Abstract
Ku70 and Ku80 form the Ku heterodimer, a ring-shaped complex that initiates the non-homologous end-joining (NHEJ) DNA repair pathway1. Ku binds to double-stranded DNA ends and recruits other NHEJ factors, including LIG4 and DNA-PKcs. Although Ku can bind to double-stranded RNA (dsRNA)2 and trap mutated DNA-PKcs on ribosomal RNA3,4, the physiological role of the Ku–RNA interaction in otherwise wild-type cells remains unclear. Notably, Ku is dispensable for mouse development5,6 but is essential in human cells7. Despite their similar genome sizes, human cells express about 100-fold more Ku than mouse cells, suggesting that Ku has functions beyond NHEJ, possibly through a dose-sensitive interaction with dsRNA, which binds Ku 10 to 100 times more weakly than double-stranded DNA2,8. Here, Ku depletion induces profound interferon and NF-κB signalling via the dsRNA sensor MDA5–RIG-I and MAVS. Prolonged Ku degradation further activates other dsRNA sensors, especially PKR (also known as EIF2AK2) (suppressing translation) and OAS–RNaseL (cleaving ribosomal RNA), leading to growth arrest and cell death. Knockout of MAVS, RIG-I or MDA5 suppressed interferon signalling and, similarly to PKR knockout, partially rescued Ku-depleted human cells. Ku crosslinking and immunoprecipitation analyses revealed binding of Ku to diverse dsRNA molecules, predominantly stem-loops in primate-specific antisense Alu elements9 in introns and 3′ untranslated regions. Ku expression is higher in primates than in non-primate mammals and is tightly correlated with Alu expansion. Thus, Ku has a vital role in accommodating Alu expansion in primates, limiting dsRNA-induced innate immunity, which explains its high expression and essential function in human cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-seq data generated in this study have been deposited into the Gene Expression Omnibus (GEO) under accession GSE294709. The Ku and DNA-PKcs irCLIP data are available via the GEO under accession GSE109026. PTBP1 and RBFOX2 CLIP data used for controls are available via GEO under accessions GSE78832 and ENCODE Data Coordination Center under accession ENCSR456FVU. RNA-seq data for human primary B cells are available at the GEO under accession GSE110669, and for mouse primary B cells under accessions GSE212194, GSE212195, GSE212196, and GSE214643. The RNA-seq data for Canis lupus and macaques are available at the GEO under accessions GSE125483, data for macaques are available at GES30352, and for macaques and Lemuroidea at Primate Expression Altas and at NHPRTR Tissue Sample Database (SPR021223). Data for Equus caballus are available at GEO accessions GSM1139274, GSE43013 and EPR006861. The human reference genome (hg19) was from https://grch37.ensembl.org/Homo_sapiens/Info/Index. Source data are provided with this paper.
References
Lieber, M. R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79, 181–211 (2010).
Yoo, S. & Dynan, W. S. Characterization of the RNA binding properties of Ku protein. Biochemistry 37, 1336–1343 (1998).
Shao, Z. et al. DNA-PKcs has KU-dependent function in rRNA processing and haematopoiesis. Nature 579, 291–296 (2020).
Jiang, W. et al. Differential phosphorylation of DNA-PKcs regulates the interplay between end-processing and end-ligation during nonhomologous end-joining. Mol. Cell 58, 172–185 (2015).
Nussenzweig, A. et al. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature 382, 551–555 (1996).
Gu, Y. et al. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity 7, 653–665 (1997).
Li, G., Nelsen, C. & Hendrickson, E. A. Ku86 is essential in human somatic cells. Proc. Natl Acad. Sci. USA 99, 832–837 (2002).
Dalby, A. B., Goodrich, K. J., Pfingsten, J. S. & Cech, T. R. RNA recognition by the DNA end-binding Ku heterodimer. RNA 19, 841–851 (2013).
Deininger, P. Alu elements: know the SINEs. Genome Biol. 12, 236 (2011).
Wang, X. S., Lee, B. J. & Zha, S. The recent advances in non-homologous end-joining through the lens of lymphocyte development. DNA Repair 94, 102874 (2020).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e516 (2017).
Jiang, W. et al. Phosphorylation at S2053 in murine (S2056 in human) DNA-PKcs is dispensable for lymphocyte development and class switch recombination. J. Immunol. 203, 178–187 (2019).
Walker, J. R., Corpina, R. A. & Goldberg, J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412, 607–614 (2001).
Chen, X. et al. Structure of an activated DNA-PK and its implications for NHEJ. Mol. Cell 81, 801–810.e803 (2021).
Pfingsten, J. S. et al. Mutually exclusive binding of telomerase RNA and DNA by Ku alters telomerase recruitment model. Cell 148, 922–932 (2012).
Chen, H. et al. Structural insights into yeast telomerase recruitment to telomeres. Cell 172, 331–343.e313 (2018).
Ting, N. S., Yu, Y., Pohorelic, B., Lees-Miller, S. P. & Beattie, T. L. Human Ku70/80 interacts directly with hTR, the RNA component of human telomerase. Nucleic Acids Res. 33, 2090–2098 (2005).
Ting, N. S., Pohorelic, B., Yu, Y., Lees-Miller, S. P. & Beattie, T. L. The human telomerase RNA component, hTR, activates the DNA-dependent protein kinase to phosphorylate heterogeneous nuclear ribonucleoprotein A1. Nucleic Acids Res. 37, 6105–6115 (2009).
Britton, S., Coates, J. & Jackson, S. P. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J. Cell Biol. 202, 579–595 (2013).
Crowe, J. L. et al. DNA-PKcs phosphorylation at the T2609 cluster alters the repair pathway choice during immunoglobulin class switch recombination. Proc. Natl Acad. Sci. USA 117, 22953–22961 (2020).
Saito, Y. & Kanemaki, M. T. Targeted protein depletion using the auxin-inducible degron 2 (AID2) system. Curr. Protoc. 1, e219 (2021).
Zhang, L., Yu, J., Park, B. H., Kinzler, K. W. & Vogelstein, B. Role of BAX in the apoptotic response to anticancer agents. Science 290, 989–992 (2000).
Menolfi, D. et al. ATR kinase supports normal proliferation in the early S phase by preventing replication resource exhaustion. Nat. Commun. 14, 3618 (2023).
Liu, X. et al. CtIP is essential for early B cell proliferation and development in mice. J. Exp. Med. 216, 1648–1663 (2019).
Platanias, L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Der, S. D., Zhou, A., Williams, B. R. & Silverman, R. H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl Acad. Sci. USA 95, 15623–15628 (1998).
Ruis, B. L., Fattah, K. R. & Hendrickson, E. A. The catalytic subunit of DNA-dependent protein kinase regulates proliferation, telomere length, and genomic stability in human somatic cells. Mol. Cell. Biol. 28, 6182–6195 (2008).
Goytisolo, F. A., Samper, E., Edmonson, S., Taccioli, G. E. & Blasco, M. A. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol.Cell Biol. 21, 3642–3651 (2001).
Zhu, Y. et al. Phosphorylation of DNA-PKcs at the S2056 cluster ensures efficient and productive lymphocyte development in XLF-deficient mice. Proc. Natl Acad. Sci. USA 120, e2221894120 (2023).
Crowe, J. L. et al. Kinase-dependent structural role of DNA-PKcs during immunoglobulin class switch recombination. Proc. Natl Acad. Sci. USA 115, 8615–8620 (2018).
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017).
Tan, X., Sun, L., Chen, J. & Chen, Z. J. Detection of microbial infections through innate immune sensing of nucleic acids. Annu. Rev. Microbiol. 72, 447–478 (2018).
Wang, Y., Ghosh, G. & Hendrickson, E. A. Ku86 represses lethal telomere deletion events in human somatic cells. Proc. Natl Acad. Sci. USA 106, 12430–12435 (2009).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Chen, Y. G. & Hur, S. Cellular origins of dsRNA, their recognition and consequences. Nat. Rev. Mol. Cell Biol. 23, 286–301 (2022).
Malathi, K., Dong, B., Gale, M. Jr. & Silverman, R. H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448, 816–819 (2007).
Dey, M. et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell 122, 901–913 (2005).
Signer, R. A., Magee, J. A., Salic, A. & Morrison, S. J. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509, 49–54 (2014).
Li, Y. et al. Ribonuclease L mediates the cell-lethal phenotype of double-stranded RNA editing enzyme ADAR1 deficiency in a human cell line. eLife 6, e25687 (2017).
Han, Y. et al. Structure of human RNase L reveals the basis for regulated RNA decay in the IFN response. Science 343, 1244–1248 (2014).
Kelly, R. D. et al. Noncanonical functions of Ku may underlie essentiality in human cells. Sci. Rep. 13, 12162 (2023).
Chung, H. et al. Human ADAR1 prevents endogenous RNA from triggering translational shutdown. Cell 172, 811–824.e814 (2018).
Hu, S. B. et al. ADAR1p150 prevents MDA5 and PKR activation via distinct mechanisms to avert fatal autoinflammation. Mol. Cell 83, 3869–3884.e3867 (2023).
Shah, A., Qian, Y., Weyn-Vanhentenryck, S. M. & Zhang, C. CLIP Tool Kit (CTK): a flexible and robust pipeline to analyze CLIP sequencing data. Bioinformatics 33, 566–567 (2017).
Eller, C. D. et al. Repetitive sequence environment distinguishes housekeeping genes. Gene 390, 153–165 (2007).
Zhang, X. O., Pratt, H. & Weng, Z. Investigating the potential roles of SINEs in the human genome. Annu. Rev. Genomics Hum. Genet. 22, 199–218 (2021).
Ahmad, S. et al. Breaching self-tolerance to Alu duplex RNA underlies MDA5-mediated inflammation. Cell 172, 797–810.e713 (2018).
Liu, G. E., Alkan, C., Jiang, L., Zhao, S. & Eichler, E. E. Comparative analysis of Alu repeats in primate genomes. Genome Res. 19, 876–885 (2009).
Crow, Y. J. & Manel, N. Aicardi–Goutieres syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Weyn-Vanhentenryck, S. M. et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep. 6, 1139–1152 (2014).
Zhang, C. & Darnell, R. B. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat. Biotechnol. 29, 607–614 (2011).
Hoffman, Y., Pilpel, Y. & Oren, M. microRNAs and Alu elements in the p53-Mdm2-Mdm4 regulatory network. J. Mol. Cell. Biol. 6, 192–197 (2014).
Dorrity, T. J. et al. Long 3′UTRs predispose neurons to inflammation by promoting immunostimulatory double-stranded RNA formation. Sci. Immunol. 8, eadg2979 (2023).
Lubelsky, Y. & Ulitsky, I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature 555, 107–111 (2018).
Burleigh, K. et al. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci. Immunol. 5, eaba4219 (2020).
Xia, B. et al. On the genetic basis of tail-loss evolution in humans and apes. Nature 626, 1042–1048 (2024).
Chen, L. L., DeCerbo, J. N. & Carmichael, G. G. Alu element-mediated gene silencing. EMBO J. 27, 1694–1705 (2008).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Yesbolatova, A., Natsume, T., Hayashi, K. I. & Kanemaki, M. T. Generation of conditional auxin-inducible degron (AID) cells and tight control of degron-fused proteins using the degradation inhibitor auxinole. Methods 164–165, 73–80 (2019).
Moriarity, B. S. et al. Modular assembly of transposon integratable multigene vectors using RecWay assembly. Nucleic Acids Res. 41, e92 (2013).
Yamamoto, K. et al. Kinase-dead ATM protein is highly oncogenic and can be preferentially targeted by topo-isomerase I inhibitors. eLife 5, e14709 (2016).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Zarnegar, B. J. et al. irCLIP platform for efficient characterization of protein–RNA interactions. Nat. Methods 13, 489–492 (2016).
Van Nostrand, E. L. et al. A large-scale binding and functional map of human RNA-binding proteins. Nature 583, 711–719 (2020).
Wu, B. et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152, 276–289 (2013).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Darty, K., Denise, A. & Ponty, Y. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics 25, 1974–1975 (2009).
Brawand, D. et al. The evolution of gene expression levels in mammalian organs. Nature 478, 343–348 (2011).
Pipes, L. et al. The non-human primate reference transcriptome resource (NHPRTR) for comparative functional genomics. Nucleic Acids Res. 41, D906–D914 (2013).
Leinonen, R., Sugawara, H., Shumway, M. & International Nucleotide Sequence Database Collaboration. The sequence read archive. Nucleic Acids Res. 39, D19–D21 (2011).
Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta 2, e107 (2023).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Putri, G. H., Anders, S., Pyl, P. T., Pimanda, J. E. & Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 38, 2943–2945 (2022).
Smit, A., Hubley, R. & Green, P. RepeatMasker Open-4.0. 2013-2015 RepeatMasker http://www.repeatmasker.org (2022).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Hofacker, I. L. Vienna RNA secondary structure server. Nucleic Acids Res. 31, 3429–3431 (2003).
Ahler, E. et al. Doxycycline alters metabolism and proliferation of human cell lines. PLoS ONE 8, e64561 (2013).
Acknowledgements
The authors thank M. Kanemaki for providing the HCT116 OsTIR1 cell line; K. Meek for providing cell lines derived from dogs and horses; R. Dalla-Favera for providing transcriptional data from human primary B cells; S. Hur and C.-Z. Zhang for sharing the irAlu and non-irAlu annotations; W. Yang, X. Wu, R. Baer and K. Basso for helpful discussions; T. Palomero for information on the JAK signalling pathway; A. Ciccia for cGAS–STING–interferon pathway detection reagents; S. Prezetocka and J. Karlseder for helpful discussion and advice about ZBP1; and S. P. Goff for advice about virus RNA stem-loops during manuscript revision. Owing to space constraints, we often cited reviews rather than original publications. We apologize to the colleagues whose original works were not cited here. Funding: Y.Z is supported by a Cancer Research Institute fellowship. The project is partially supported by US National Institutes of Health (NIH) grants R01CA275184, R01CA158073, R01CA271595 and P01CA174653 to S.Z.; R35GM145279 to C.Z.; R01NS127802 to H.C.; R35GM150778 and R21AI171827 to A.L.-S.; R01CA266524 to E.A.H.; and R35CA253126 to R.R. A.P. and M.M. were supported by Agence Nationale de la Recherche (ANR AAPG2023-PRC–XXL). S.Z. was a Leukemia Lymphoma Society Scholar. J.A.G. was supported by NIH grant T32GM145440, and T.J.D. was supported by T32AR076953. C.Z. received a Scientific Innovation Award from Brain Research Foundation. H.C received the MIND prize, supported by The Pershing Square Foundation, B. Ackman and N. Oxman. C.S.-P. was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (2018-05518) and the Discovery Accelerator Supplements program (2018-522665), and D.R.E. was funded by a Discovery Grant (RGPIN-2022-05459). This research used facilities partly funded through the NIH National Cancer Institute Cancer Center Support Grant P30CA013696 to the Herbert Irving Comprehensive Cancer Center of Columbia University. The DepMap project is partially funded by CTD2, the Achilles consortium, and The Carlos Slim Foundation in Mexico through the Slim Initiative for Genomic Medicine.
Author information
Authors and Affiliations
Contributions
Y.Z. generated and characterized the Ku–AID clones and MAVS, RIG-I, MDA5 and PKR knockout subclones, and collected and analysed RNA and protein samples. A.L. assisted with generating the LIG4–AID control cells and performed the qPCR analyses. S.M. initiated the irCLIP data analysis. B.J.L. conducted RNA-seq analyses, generated all related plots, and guided A.L. through the qPCR analyses. S.M.K. performed and analysed the in vitro Ku binding assays. J.A.G. conducted repeat J2 antibody staining and control experiments. T.J.D. performed the initial J2 staining. J.W. analysed and plotted Alu copy number and XRCC5 and XRCC6 expression across primates. K.J.W. carried out the AlphaFold 3 modelling and generated all related figures. A.P. purified human Ku protein for in vitro binding assays. D.F.M. performed preliminary RNA-seq analyses and reanalysed irCLIP data. R.D.K. generated the HEK293 Ku70-deficient cell lines in the C.S.-P. laboratory. A.B.H. developed the software used for RNA-seq data analysis and visualization. R.R. supervised J.W. on evolutionary analyses. D.R.E. and C.S.-P. supervised R.D.K in generating and initial characterization of the HEK293 Ku70-deficient cell lines. M.M. supervised A.P. for the purification of Ku protein. A.L.-S. supervised S.M.K. and advised on the in vitro binding assays. E.A.H. provided the Ku86fl/−:creERT2 and Ku86fl/− HCT116 control cell lines. H.C. supervised J.A.G. and T.J.D., provided insights into experimental design and innate immune response characterization, and contributed to data interpretation. C.Z. supervised the irCLIP analyses, identified Alu element enrichment, and developed the analysis software. S.Z. conceived the project, oversaw data analysis, and wrote the initial draft with contributions from Y.Z. and other co-authors. The Zha group uncovered the role of Ku in suppressing RNA-mediated innate immune responses and generated the Ku-irCLIP data. The Zhang laboratory conducted irCLIP data analyses and uncovered the preferential binding of Ku to Alu elements.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Sergey Iordanskiy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Ku, but not LIG4/XRCC4, is required for the viability of human cells and is highly expressed in human cells.
a-d, The Dependency Map analyses of Ku and control genes. XRCC5/Ku80 (a) and XRCC6/Ku70 (b) are essential for over 1000 human cancer cell lines tested via CRISPR KO (blue) and RNAi knockdown (purple) screens. Effect score = 0 (neutral, black line) and < −1 (essential, red dashed line) are marked. LIG4 (c) and DNA-PKcs (d) are not commonly essential in human cells tested. e-g, The mRNA expression (measured by TPM) for XRCC5 (e), XRCC6 (f), and XRCC4 (g), the obligatory partner of LIG4, in primary human (n = 3) versus mouse (n = 6) B cells. The bars represent means and SEMs from biologically independent samples. Unpaired two-tailed Student’s t-test: p < 0.0001 ****; p > 0.05 n.s. h, The essentiality of Ku in human cells is independent of TERT expression in human cancer cells. Three cancer cell lines (marked by triangles, U2OS, SAOS2, and CAL72) that are known to use ALT to extend their telomere also require Ku for survival. HCT116 is also marked for reference. Data are from the Depmap project.
Extended Data Fig. 2 Generation of Ku80-AID and LIG4-AID tagged cell lines.
a, The strategy for introducing an AID-tag at the N-terminal of endogenous Ku80 or LIG4 with a short linker. The primers used for screens are marked in the diagram and listed in supplementary methods. b, The PCR screen of homozygously tagged Ku80 clones 1, 7, and 9 with controls. c, The PCR screen of homozygously tagged LIG4 clones 7, 8 and 9 with controls. d, Western blotting shows the successful depletion of LIG4 after IAA and Dox treatment. The AID-tagged LIG4 migrated slightly slower than the untagged LIG4. e, Western blotting shows that Ku levels remain low for at least 9 days after IAA and Dox treatments. f, Representative flow cytometry analyses for the apoptosis marker Annexin V and PI after 6 days of IAA and Dox treatment. g, The Annexin V positive cell percentage is plotted at different time points. The bars represent the mean and standard deviation of n = 4 (n = 8 for the control) samples from two measurements on two independently derived cell lines. The p-values were calculated via a two-way ANOVA test with Dunnett’s correction for multiple comparisons: p < 0.0001 ****; p > 0.05 n.s. h, Representative flow cytometry plot for cell cycle analyses at 48 hr after inducing Ku degradation. S phase percentage was measured by BrdU positivity. The mid-S phase cells were gated, and their BrdU levels were plotted using a histogram. BrdU-negative S phase cells indicate replication stalling. i, Quantification of BrdU+/S phase percentage in DMSO versus IAA&Dox-treated Ku80-AID clones. The bars represent the mean and standard deviation of n = 4 samples from two measurements on two independently derived cell lines. Unpaired two-tailed Student’s t-test: p = 0.0050 **.
Extended Data Fig. 3 Ku degradation induces IFN signaling.
a and b, The gene set enrichment analyses (GSEA) for the interferon (a) and NF-kB (b)-pathways at 4 days after Ku degradation in cycling cells. c, The GSEA for the IFN pathway at 24 hr after Ku deletion in cycling cells. d, Pathway analyses of significantly downregulated genes upon Ku-degradation (24 hr). The p-values were calculated using the GOenrich function from the clusterProfiler package in R, with a list of downregulated genes as the input.
Extended Data Fig. 4 Ku degradation induces IFN signaling independent of cycling.
a, Heatmap confirms the G1 arrest after CDK4/6 inhibitor treatment, evidenced by the loss of mitotic and DNA-replication-associated genes (e.g., MCMs and DNA polymerases). The color gradient reflects the Z-scores calculated from normalized TPM across the displayed samples: red represents higher expression levels relative to the mean, while blue indicates lower expression levels. b and c, The GSEA for the interferon-pathway at 48 hr (2 days, b) and 96 hr (4 days, c) after Ku deletion in G1 arrested cells. d, The GSEA for NF-kB pathway at 4 days after Ku-degradation in G1 arrested cells. e, f and g, Quantitative RT-PCR analyses of NF-kB target genes (GADD45B (e); Jun (f); and PPP1R15A (g)). Parental HCT116 of the AID system cells (n = 9), three Ku80-AID clones (#1, 7, 9; n = 27 in (e) and 9 in (f-g)) and three HEK293 clones with inducible Ku70 (Sa11, SB, and TI; n = 18 in (e) and 27 in (f-g)) were analyzed individually and plotted together. The mRNA levels were normalized against β-actin. Bars indicate the mean ± SEM from at least three biologically independent samples. Two-way ANOVA test with Sidak’s correction for multiple comparisons: p < 0.0001 ****; p > 0.05 n.s. h, Quantification of NF-kB p50/p105 ratio from parental HCT116 cells (n = 7), Ku80-AID clones (n = 8) and LIG4-AID clones (n = 3). The bars represent mean ± SD from n ≥ 3 biologically independent samples. Unpaired two-tailed t-test with Welch’s correction, p = 0.0022 **; p > 0.05 n.s.
Extended Data Fig. 5 Ku deletion in HCT116 also induces IFN signaling.
a, PCR analyses show the specific and efficient deletion of the Ku86 flox allele upon 4OHT treatment. b, Progressive loss of viability in 4OHT treated Ku86Flox/−:CreERT2, but not control Ku86Flox/− cells. n = 6 from two independent experiments. Bars represent mean ± SD. The p-values were calculated via a two-way ANOVA test with Dunnett’s correction for multiple comparisons: p < 0.0001 ****; p > 0.05 n.s. c, Pathway analyses of significantly up-regulated genes upon Ku-deletion (96 hr). d and e, The GSEA for the interferon-pathway at 96 hr (4 days) after 4OHT treatment in control Ku86flox/− cells (d) or Ku86flox/−:CreERT2 (e). f, Heatmap of selective NF-kB targets in Ku-AID and Ku-flox cells. The color gradient reflects the Z-scores calculated from normalized TPM across the displayed samples: red represents higher expression levels relative to the mean, while blue indicates lower expression levels.
Extended Data Fig. 6 MAVS but not c-GAS-STING mediates IFN activation upon Ku degradation.
a, Western blotting shows that cGAS protein is not expressed in HCT116 cells with Hela cells as the positive control. b, Ku is required in human cells regardless of cGAS expression levels. The mRNA levels of cGAS, a dsDNA sensor provided by the Dependency Map database, were plotted against the XRCC5/Ku80 Gene deletion effect. Effect score =0 (neutral) and <−1 (essential). c-g, Quantitative RT-PCR analyses of IFN targets, ISG15 (c), IFIT1 (d), IFNβ (e), STAT1 (f), and NF-kB target GADD45B (g) validated the critical role of MAVS in mediating IFN activation following Ku-degradation. Parental HCT116 of the AID system cells (n = 9), three independently derived Ku80-AID clones (n = 18 in (c) and (f), 27 in (d-e) and (g)) and three independent MAVS KO clones (n = 18 in (c) and (f), 27 in (d-e) and (g)) were analyzed individually and plotted together. Poly I:C transfection (100 ng/ml, 24 hr) was used as a positive control (n = 9). Bars indicate the mean ± SEM from at least three biologically independent samples. Two-way ANOVA test with Sidak’s correction for multiple comparisons: p < 0.0001 ****; p > 0.05 n.s. h, Representative western blotting analyses of IFN pathway markers in multiple independently derived MAVS, RIG-I, and MDA5 KO clones. Additional clones can be found in Fig. 3a. i, Western blotting analyses of dsRNA sensors RIG-I, MDA5, MAVS, and IRF7 in HCT116, HEK293 and control Hela cells.
Extended Data Fig. 7 Ku depletion induces PKR and OAS activation beyond IFN.
a, IFNβ (up to 40 ng/ml) decrease in viability ~10% in 3 days. b. Poly I:C of 10 ng/ml and 100 ng/ml causes 40% and 99% loss of viability, regardless of Ku-AID tag (no IAA or Dox). The bars represent the mean and SEM from multiple repeats. c, Ku80-AID cells showed increased OP-puro low populations after IAA and Dox treatment, suggesting reduced translation. Bars represent mean ± SD of n = 3 samples from three independent clones. Two-way ANOVA test with Sidak’s correction for multiple comparisons: p < 0.0001 ****. Dox alone slightly decreased OP-puro in parental AID cells, consistent with its mild effect on mitochondrial translation79. To confirm the translation defect was Ku-specific, 4OHT-treated Ku86flox/−:CreERT2 and Ku86flox/− controls were tested and showed minimal background changes (Fig. 3c). d, Multiple members of the OAS family are transcriptionally induced (up to 128-fold for OAS1) upon Ku degradation (day 4 after Ku degradation). The error bars represent the standard errors from three independently derived cell lines. To plot all the OAS family genes with varying expression levels on the same plot, the Log2 fold changes were calculated. The untreated sample, with a Log2FC of 0, was excluded from the plot. Thus, no brackets can be drawn. e, The 28S versus 5S and 5.8S rRNA ratios in DMSO-treated cells (Ctrl, n = 5) versus IAA&Dox-treated Ku80-AID cells (n = 5 for CDK4/6i treated samples, otherwise n = 3), and poly I:C (100 ng/ml) transfected cells (n = 3). f, The representative histogram shows the relative reduction of 28S size and the relative decrease of 28S rRNA abundance after Ku80 degradation. g, The 28S versus 18S rRNA ratio in 4OHT treated control Ku86flox/− cells and Ku86flox/−:CreERT2 cells (Day7). For panel d, e, and g, at least three independent samples were measured at each time point from multiple clones. The means and SDs were plotted. The p-values were calculated via an unpaired two-tailed Student’s t-test, p < 0.0001 ****; p > 0.05 n.s.
Extended Data Fig. 8 Ku deletion in HEK293 cells induces NF-kB signaling and PKR and RNaseL activation.
a, Dox (1 µg/ml) induces ~10 fold Ku70 mRNA overexpression the engineered HEK293 cells. CRISPR KO only removed a small exon fragment from endogenous Ku70, therefore, even after Dox is withdrawn, RNA-seq can still detect endogenous Ku70 mRNA (although it is not able to encode Ku70 protein). The bars represent means and SEMs from n ≥ 3 biologically independent samples. Unpaired two-tailed Student’s t-test: p < 0.0001 ****. b, Western blot shows the slow loss of Ku, the robust NF-kB and PKR activation, in HEK293 cells after Dox withdrawal. c, The moderate and significant loss of viability upon Dox withdrawal in HEK293 cells. n = 9 from three independent experiments. Bars represent mean ± SD. The p-values were calculated via a two-way ANOVA test with Sidak’s correction for multiple comparisons: p < 0.0001 ****. d Pathway analyses of significantly upregulated genes upon Ku-depletion in HEK293 cells (8 days). e, The GSEA for the NF-kB-pathway at 8 days after Dox was withdrawn. Three independently derived clones were analyzed and plotted together. f, The HEK293 cells do not express IRF7 mRNA. See Extended Data Fig. 6i for data support that IRF7 protein also can not be induced in the HEK293 after IFN treatment. The bars represent means and SEMs from n = 26 (HCT116) and 9 (HEK293) biologically independent samples. Unpaired two-tailed t-test with Welch’s correction: p < 0.0001 ****. g, Gating strategy and quantification of protein translation by OP-Puro in HEK293 cells after Dox withdrawal at 6 days, two independent clones (Sa11 and SB) with n = 3 independent measurements. Bars represent mean ± SD. Two-way ANOVA test with Sidak’s correction for multiple comparisons: p < 0.0001 ****; p > 0.05 n.s. h, The 28S versus 5S and 5.8S rRNA ratio in Dox-treated and Dox withdrawal (8 days) HEK293 cells with inducible Ku70 expression. n = 3 independent samples were measured from multiple clones. The means and SDs were plotted. The p-values were calculated via an unpaired two-tailed Student’s t-test, p = 0.0037 **.
Extended Data Fig. 9 CRISPR KO of MAVS, RIG-I, and PKR and higher primates have increased Ku expression.
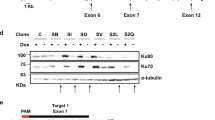
a, The validation of PKR CRISPR KO. PKR is an IFN inducible gene. The poly I:C treatment induced maximal PKR expression and activation only in parental cells and ensured a clean CRISPR KO. b, Western blotting analyses of Ku in human, non-human primates, and non-primate cell lines. c-e, The mRNA expression levels of XRCC5(c), XRCC6(d), and ADAR1(e) were analyzed across species. Human and mouse data came from our previous studies, while other species’ data were from public datasets. The bars represent means and SEMs from n = 26, 25, 17, 18, 22 and 20 (c-d) and n = 36 and 20 (e) biologically independent samples (n values are in the same order as plotted). f-h, AlphaFold3 modeling of core human Ku (XRCC5 aa 1–536, pink/purple; XRCC6 aa 36–521, yellow/orange) bound to asSL1 RNA of Alu (70nt, silver) or perfectly paired dsDNA (58nt, gold). In each model, light-shaded proteins (pink and yellow) represent Ku bound to asSL1 RNA, while darker-shaded proteins (purple and orange) represent Ku bound to dsDNA. The top view (f) and front-side view (g) are presented in overlay format. The inset shows that base U19 and U21 of asSL1 flip out in the Ku-bound model but not in the absence of Ku. Panel (h) compares the structures of Ku-bound dsDNA (top, Ku hidden), Ku-bound asSL1 RNA (middle, Ku hidden), and free asSL1 RNA. The hairpin diagram was generated using the IDT Oligo Analysis Tool. The U19 and U21 bases of asSL1 flip out to accommodate Ku binding. See Methods for further details.
Extended Data Fig. 10 Ku binds to SINEs without selective preference for a specific Alu subfamily.
a-b, Two other nuclear RBPs are shown to compare to Ku and DNA-PKcs, highlighting the strong preference for introns and repetitive elements for Ku and DNA-PKcs. c-d, The genomic distribution of DNA-PKcs irCLIP tags (c) and overlap with repetitive elements (d). e-f, as Alu elements of specific subfamilies with the numbers overlapping with Ku (e) and DNA-PKcs (f) irCLIP peaks shown in the y-axis and the total numbers in the human genome shown in the x-axis. The red arrows indicate Alu subfamilies with lower-than-expected irCLIP signal.
Extended Data Fig. 11 Ku binds to monomeric Alu at the stem loops.
a-b, The relative fraction of irAlu and non-irAlu defined by Wu et al. Cell 201867 bound by Ku (a) or DNA-PKcs (b). c, The distribution of LINE element orientation among the Ku and DNA-PKcs irCLIP sites. d-e, The irCLIP sites preference of Ku (d) and DNA-PKcs (e) in asAlu elements. The red is a higher frequency. f, The impact of irCLIP-Ku on mRNA expression. The Log2 (mean TPM + 1) in DMSO versus IAA-and Dox- treated (day 4) Ku80-AID samples were plotted. The red and blue dots are genes with irCLIP-Ku in 3′ UTR (with or without intron, red), or in intron only (blue) g, The impact of Alu on mRNA expression after Ku degradation. The Log2 (mean TPM + 1) in DMSO versus IAA- and Dox-treated (day 4) Ku80-AID samples were plotted. The red and blue dots are genes with annotated Alus in 3′ UTR (with or without Intron, red, n > 2), or in Intron only (blue, n > 9). The data associated with panels f and g can be found in SI Tab. 12 and 13.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and legends for Supplementary Tables 1–15 (tables supplied separately).
Supplementary Tables
Supplementary Tables 1–15 – see Supplementary Information descriptions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Li, A., Maji, S. et al. Ku limits RNA-induced innate immunity to allow Alu expansion in primates. Nature 643, 562–571 (2025). https://doi.org/10.1038/s41586-025-09104-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09104-w