Abstract
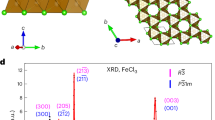
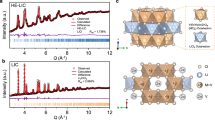
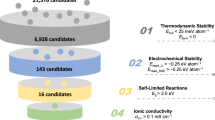
All-solid-state batteries require advanced cathode designs to realize their potential for high energy density and economic viability1,2,3. Integrated all-in-one cathodes, which eliminate inactive conductive additives and heterogeneous interfaces, hold promise for substantial energy and stability gains but are hindered by materials lacking sufficient Li+/e− conductivity, mechanical robustness and structural stability4,5,6,7,8,9,10,11,12,13,14. Here we present Li1.3Fe1.2Cl4, a cost-effective halide material that overcomes these challenges. Leveraging reversible Fe2+/Fe3+ redox and rapid Li+/e− transport within its framework, Li1.3Fe1.2Cl4 achieves an electrode energy density of 529.3 Wh kg−1 versus Li+/Li. Critically, Li1.3Fe1.2Cl4 shows unique dynamic properties during cycling, including reversible local Fe migration and a brittle-to-ductile transition that confers self-healing behaviour. This enables exceptional cycling stability, maintaining 90% capacity retention for 3,000 cycles at a rate of 5 C. Integration of Li1.3Fe1.2Cl4 with a nickel-rich layered oxide further increases the energy density to 725.6 Wh kg−1. By harnessing the advantageous dynamic mechanical and diffusion properties of all-in-one halides, this work establishes all-in-one halides as an avenue for energy-dense, durable cathodes in next-generation all-solid-state batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are provided in this paper (and its supplementary files). Source data are provided with this paper.
References
Balaish, M. et al. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 6, 227–239 (2021).
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Schmuch, R., Wagner, R., Hörpel, G., Placke, T. & Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Inoishi, A., Nishio, A., Yoshioka, Y., Kitajou, A. & Okada, S. A single-phase all-solid-state lithium battery based on Li1.5Cr0.5Ti1.5(PO4)3 for high rate capability and low temperature operation. Chem. Commun. 54, 3178–3181 (2018).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Li, M. et al. Dense all-electrochem-active electrodes for all-solid-state lithium batteries. Adv. Mater. 33, 2008723 (2021).
Han, F., Gao, T., Zhu, Y., Gaskell, K. J. & Wang, C. A battery made from a single material. Adv. Mater. 27, 3473–3483 (2015).
Wang, K., Gu, Z., Xi, Z., Hu, L. & Ma, C. Li3TiCl6 as ionic conductive and compressible positive electrode active material for all-solid-state lithium-based batteries. Nat. Commun. 14, 1396 (2023).
Inoishi, A., Omuta, T., Kobayashi, E., Kitajou, A. & Okada, S. A single-phase, all-solid-state sodium battery using Na3−xV2−xZrx(PO4)3 as the cathode, anode, and electrolyte. Adv. Mater. Interfaces 4, 1600942 (2017).
Inoishi, A. et al. Single-phase all-solid-state lithium-ion battery using Li3V2(PO4)3 as the cathode, anode, and electrolyte. ChemistrySelect 2, 7925–7929 (2017).
Tan, D. H. S. et al. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte. ACS Energy Lett. 4, 2418–2427 (2019).
Ohno, S., Rosenbach, C., Dewald, G. F., Janek, J. & Zeier, W. G. Linking solid electrolyte degradation to charge carrier transport in the thiophosphate‐based composite cathode toward solid‐state lithium-sulfur batteries. Adv. Funct. Mater. 31, 2010620 (2021).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Cui, L. et al. A cathode homogenization strategy for enabling long-cycle-life all-solid-state lithium batteries. Nat. Energy 9, 1084–1094 (2024).
Minnmann, P., Quillmann, L., Burkhardt, S., Richter, F. H. & Janek, J. Quantifying the impact of charge transport bottlenecks in composite cathodes of all-solid-state batteries. J. Electrochem. Soc. 168, 040537 (2021).
Bielefeld, A., Weber, D. A. & Janek, J. Microstructural modeling of composite cathodes for all-solid-state batteries. J. Phys. Chem. C 123, 1626–1634 (2019).
Strauss, F. et al. Impact of cathode material particle size on the capacity of bulk-type all-solid-state batteries. ACS Energy Lett. 3, 992–996 (2018).
Minnmann, P. et al. Designing cathodes and cathode active materials for solid‐state batteries. Adv. Energy Mater. 12, 2201425 (2022).
Cao, D. et al. Processing strategies to improve cell-level energy density of metal sulfide electrolyte-based all-solid-state Li metal batteries and beyond. ACS Energy Lett. 5, 3468–3489 (2020).
Wang, C. et al. Interface-assisted in-situ growth of halide electrolytes eliminating interfacial challenges of all-inorganic solid-state batteries. Nano Energy 76, 105015 (2020).
Zahnow, J. et al. Impedance analysis of NCM cathode materials: electronic and ionic partial conductivities and the influence of microstructure. ACS Appl. Energy Mater. 4, 1335–1345 (2021).
Tian, Y. et al. Compatibility issues between electrodes and electrolytes in solid-state batteries. Energy Environ. Sci. 10, 1150–1166 (2017).
Zhang, J. et al. Challenges and strategies of low-pressure all-solid-state batteries. Adv. Mater. 37, 2413499 (2025).
Hu, X. et al. External-pressure–electrochemistry coupling in solid-state lithium metal batteries. Nat. Rev. Mater. 9, 305–320 (2024).
Zhou, J. et al. Healable and conductive sulfur iodide for solid-state Li–S batteries. Nature 627, 301–305 (2024).
Swamy, T., Chen, X. & Chiang, Y.-M. Electrochemical redox behavior of Li ion conducting sulfide solid electrolytes. Chem. Mater. 31, 707–713 (2019).
Shao, B. et al. Enabling conversion‐type iron fluoride cathode by halide‐based solid electrolyte. Adv. Funct. Mater. 32, 2206845 (2022).
Liang, J. et al. Halide layer cathodes for compatible and fast-charged halides-based all-solid-state Li metal batteries. Angew. Chem. Int. Ed. 62, e202217081 (2023).
Liu, Z. et al. Low-cost iron trichloride cathode for all-solid-state lithium-ion batteries. Nat. Sustain. 7, 1492–1500 (2024).
Liu, Z., Zhang, G., Pepas, J., Ma, Y. & Chen, H. Li2FeCl4 as a cost-effective and durable cathode for solid-state Li-ion batteries. ACS Energy Lett. 9, 5464–5470 (2024).
Fu, J. et al. Superionic conducting halide frameworks enabled by interface-bonded halides. J. Am. Chem. Soc. 145, 2183–2194 (2023).
Li, X. et al. Structural regulation of halide superionic conductors for all-solid-state lithium batteries. Nat. Commun. 15, 53 (2024).
Wang, Q. et al. Designing lithium halide solid electrolytes. Nat. Commun. 15, 1050 (2024).
Jun, K., Chen, Y., Wei, G., Yang, X. & Ceder, G. Diffusion mechanisms of fast lithium-ion conductors. Nat. Rev. Mater. 9, 887–905 (2024).
Kanno, R. et al. Structure, ionic conductivity, and phase transformation in new polymorphs of the double chloride spinel, Li2FeCl4. J. Solid State Chem. 72, 363–375 (1988).
Tanibata, N. et al. High formability and fast lithium diffusivity in metastable spinel chloride for rechargeable all-solid-state lithium-ion batteries. Adv. Energy Sustain. Res. 1, 2000025 (2020).
Hebb, M. H. Electrical conductivity of silver sulfide. J. Chem. Phys. 20, 185–190 (1952).
Stallard, J. C. et al. Mechanical properties of cathode materials for lithium-ion batteries. Joule 6, 984–1007 (2022).
Qu, M. et al. Nanomechanical quantification of elastic, plastic, and fracture properties of LiCoO2. Adv. Energy Mater. 2, 940–944 (2012).
Xu, R., Sun, H., de Vasconcelos, L. S. & Zhao, K. Mechanical and structural degradation of LiNixMnyCozO2 cathode in Li-ion batteries: an experimental study. J. Electrochem. Soc. 164, A3333–A3341 (2017).
Liu, H. et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes. Science 344, 1252817 (2014).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Jain, A. et al. The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Wang, S. et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew. Chem. Int. Ed. 58, 8039–8043 (2019).
Wang, S., Liu, Y. & Mo, Y. Frustration in super‐ionic conductors unraveled by the density of atomistic states. Angew. Chem. 62, e202215544 (2023).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Farrow, C. et al. PDFfit2 and PDFgui: computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 19, 335219 (2007).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Munoz, M., Argoul, P. & Farges, F. Continuous Cauchy wavelet transform analyses of EXAFS spectra: a qualitative approach. Am. Mineral. 88, 694–700 (2003).
Lee, Y.-G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020).
Acknowledgements
We thank the support from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Research Chair Program (CRC), the Canada Foundation for Innovation (CFI), and Western University. The synchrotron-related characterizations were completed at the HXMA, SXRMB, BXDS and SGM beamline at the Canadian Light Source (CLS), which is supported by the Canadian Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the National Research Council (NRC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan, as well as the BL02B02 (31124.02.SSRF.BL02B02) beamline of the Shanghai Synchrotron Radiation Facility (SSRF), which is supported by the ME2 project under contract from National Natural Science Foundation of China (11227902). We thank S. Xia and the In-Situ Center for Physical Sciences of Shanghai Jiao Tong University for the assistance with the SEM measurements. J.F. acknowledges the support from the programme of the China Scholarships Council. C.W. acknowledges the Banting Postdoctoral Fellowship (BPF-180162). X.S. and C.W. appreciate the funding support from the National Natural Science Foundation of China (grant nos. W2441017, 22409103), the “Innovation Yongjiang 2035” Key R&D Program (grant nos. 2024Z040, 2025Z063). Part of this work was conducted at the NOMAD beamlines at ORNL’s Spallation Neutron Source, which was sponsored by the Scientific User Facilities Division, Office of Basic Sciences, US Department of Energy. Y.M. acknowledges the support from the National Science Foundation Award 2004837 and the computational facilities from the University of Maryland supercomputing resources.
Author information
Authors and Affiliations
Contributions
J.F. designed the experiments and carried out the sample synthesis and most of the characterizations. S.W. performed the computational simulations (under the supervision of Y.M.). C.W. guided the writing of the paper, the fabrication of pouch cells and the design of schematic figures. J.W.R. helped with all structural analyses of diffraction experiments. Y.Z. and X.L. helped with electron microscope-related experiments. W.L., J.T.K., Y.H., X.H. and Y.G. carried out the synchrotron-related measurements. J. Liu performed neutron data collection and analyses. B.F. and H.A. helped with nanoindentation tests. H.S., J. Liang, X.Y. and F.Z. helped with interpreting and organizing the data. Z.W. and S.Z. helped with the battery and DMA testing. J.F. and J. Luo discussed and wrote the paper. T.-K.S., Y.M. and X.S. supervised the project. All the authors helped to revise the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Laidong Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–44, Tables 1–12 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, J., Wang, C., Wang, S. et al. A cost-effective all-in-one halide material for all-solid-state batteries. Nature 643, 111–118 (2025). https://doi.org/10.1038/s41586-025-09153-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09153-1
This article is cited by
-
A solid electrolyte with active stability
Nature Materials (2025)