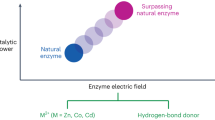
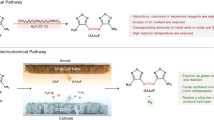
Abstract
Electrochemistry is undergoing a resurgence in synthetic chemistry and has compelling advantages1. Repurposing natural enzymes through synthetic chemical strategies holds promise for exploring new chemical space2,3,4,5,6. Elegant strategies, including directed evolution7,8,9,10, artificial enzymes11 and photoenzymatic catalysis12,13, have demonstrated their capacities for expanding the applications of enzymes in both academia and industry. However, the integration of electrochemistry with enzymes has primarily been limited to replicating previously established enzyme functions14,15,16. Key challenges in achieving new enzyme reactivity with electricity include compatibility issues and difficulties in heterogeneous electron transfer. Here we report the reshaping of thiamine-dependent enzymes with ferrocene-mediated electrocatalysis to unlock an unnatural dynamic kinetic oxidation of α-branched aldehydes. This robust electroenzymatic approach yields various bioactive (S)-profens with up to 99% enantiomeric excess; it is applicable with whole cells overexpressing the enzyme and using down to 0.05 mol% enzyme loadings. Mechanistic investigations show multiple functions of the electroenzyme in precise substrate discrimination, accelerating racemization and facilitating kinetically matched electron transfer events.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information.
References
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2, 409–421 (2018).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Buller, R. et al. From nature to industry: harnessing enzymes for biocatalysis. Science 382, eadh8615 (2023).
Kissman, E. N. et al. Expanding chemistry through in vitro and in vivo biocatalysis. Nature 631, 37–48 (2024).
Reetz, M. T., Qu, G. & Sun, Z. Engineered enzymes for the synthesis of pharmaceuticals and other high-value products. Nat. Synth. 3, 19–32 (2024).
Arnold, F. H. Directed evolution: bringing new chemistry to life. Angew. Chem. Int. Ed. 57, 4143–4148 (2018).
Bornscheuer, U. T., Hauer, B., Jaeger, K. E. & Schwaneberg, U. Directed evolution empowered redesign of natural proteins for the sustainable production of chemicals and pharmaceuticals. Angew. Chem. Int. Ed. 58, 36–40 (2019).
Qu, G., Li, A., Acevedo-Rocha, C. G., Sun, Z. & Reetz, M. T. The crucial role of methodology development in directed evolution of selective enzymes. Angew. Chem. Int. Ed. 59, 13204–13231 (2020).
Wang, Y. et al. Directed evolution: methodologies and applications. Chem. Rev. 121, 12384–12444 (2021).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Chen, H. et al. Fundamentals, applications, and future directions of bioelectrocatalysis. Chem. Rev. 120, 12903–12993 (2020).
Boucher, D. G. et al. Bioelectrocatalytic synthesis: concepts and applications. Angew. Chem. Int. Ed. 62, e202307780 (2023).
Lin, Y., Yu, J. & Ye, K.-Y. Integrating electrocatalysis with biocatalysis for asymmetric synthesis. Org. Chem. Front. 11, 7243–7248 (2024).
Hollmann, F., Hofstetter, K., Habicher, T., Hauer, B. & Schmid, A. Direct electrochemical regeneration of monooxygenase subunits for biocatalytic asymmetric epoxidation. J. Am. Chem. Soc. 127, 6540–6541 (2005).
Chen, H. et al. One-pot bioelectrocatalytic conversion of chemically inert hydrocarbons to imines. J. Am. Chem. Soc. 144, 4047–4056 (2022).
Wu, R. et al. Enzymatic electrosynthesis of glycine from CO2 and NH3. Angew. Chem. Int. Ed. 62, e202218387 (2023).
Jayathilake, B. S., Bhattacharya, S., Vaidehi, N. & Narayanan, S. R. Efficient and selective electrochemically driven enzyme-catalyzed reduction of carbon dioxide to formate using formate dehydrogenase and an artificial cofactor. Acc. Chem. Res. 52, 676–685 (2019).
Castañeda-Losada, L. et al. Bioelectrocatalytic cofactor regeneration coupled to CO2 fixation in a redox-active hydrogel for stereoselective C−C bond formation. Angew. Chem. Int. Ed. 60, 21056–21061 (2021).
Chen, H. et al. Upgraded bioelectrocatalytic N2 fixation: from N2 to chiral amine intermediates. J. Am. Chem. Soc. 141, 4963–4971 (2019).
Long, C.-J. et al. Merging the non-natural catalytic activity of lipase and electrosynthesis: asymmetric oxidative cross-coupling of secondary amines with ketones. Angew. Chem. Int. Ed. 61, e202203666 (2022).
Duan, X. et al. Deracemization of sulfoxides combining electrocatalysis and biocatalysis. Chin. J. Chem. 42, 3107–3112 (2024).
Ruccolo, S. et al. Electrochemical recycling of adenosine triphosphate in biocatalytic reaction cascades. J. Am. Chem. Soc. 144, 22582–22588 (2022).
Hailes, H. C. et al. Engineering stereoselectivity of ThDP-dependent enzymes. FEBS J. 280, 6374–6394 (2013).
Chen, X. et al. Intramolecular stereoselective Stetter reaction catalyzed by benzaldehyde lyase. Angew. Chem. Int. Ed. 60, 9326–9329 (2021).
Xu, Y. et al. A light-driven enzymatic enantioselective radical acylation. Nature 625, 74–78 (2024).
Xing, Z. et al. Synergistic photobiocatalysis for enantioselective triple-radical sorting. Nature 637, 1118–1123 (2025).
Liu, X., Xu, S., Chen, H. & Yang, Y. Unnatural thiamine radical enzymes for photobiocatalytic asymmetric alkylation of benzaldehydes and α-ketoacids. ACS Catal. 14, 9144–9150 (2024).
Berthold, C. L. et al. Structure of the branched-chain keto acid decarboxylase (KdcA) from Lactococcus lactis provides insights into the structural basis for the chemoselective and enantioselective carboligation reaction. Acta Crystallogr. D Biol. Crystallogr. 63, 1217–1224 (2007).
Brandt, G. S. et al. Probing the active center of benzaldehyde lyase with substitutions and the pseudosubstrate analogue benzoylphosphonic acid methyl ester. Biochem. 47, 7734–7743 (2008).
Kourist, R., Domínguez de María, P. & Miyamoto, K. Biocatalytic strategies for the asymmetric synthesis of profens – recent trends and developments. Green Chem. 13, 2607–2618 (2011).
Huerta, F. F., Minidis, A. B. E. & Bäckvall, J.-E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 30, 321–331 (2001).
Chabrière, E. et al. Crystal structure of the free radical intermediate of pyruvate:ferredoxin oxidoreductase. Science 294, 2559–2563 (2001).
Acknowledgements
We thank Y. Yang from NKU, S. Lu and Q. Dai from CIQTEK for their help on pulsed EPR experiments (CIQTEK EPR100); Y.-T. Long, W.-Z. Xie, X. Yang and C. Su from NJU for their help in mechanistic studies; H.-C. Xu, B. Wang and H. Huo from XMU for their discussions; D. Zhu from TIB for the gift of the plasmid for PaBAL; Y. Hao, Y. Zhang and S. Zhang from our group for their assistance with substrate synthesis. We thank the staff members of the Electron Spin Resonance System (https://cstr.cn/31125.02.SHMFF.ESR) at the Steady High Magnetic Field Facility, CAS (https://cstr.cn/31125.02.SHMFF). This work was supported by the National Key Research and Development Program of China 2022YFA0913000 (X.H.), the National Natural Science Foundation of China (22225703 to Y.Z., 22277053 to X.H., 224B2705 to B.Z., 223B2703 to Y.X. and 22437005 to Q.Z.), the Fundamental Research Funds for the Central Universities (0205/14380351 and 0205/14380346 to X.H.), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB0960201 and XDB0540000 to L.Y.) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2022455 to L.Y.).
Author information
Authors and Affiliations
Contributions
B.Z. developed the electroenzymatic catalysis. B.Z. and Y.X. performed most of the synthetic and mechanistic experiments. Q.Z. performed molecular dynamics simulations. A.L. and L.Y. contributed to EPR experiments. X.P. and T.Z. performed parts of the synthetic experiments and mechanistic investigations. Y.Z. assisted in mechanistic investigations and data analysis. X.H., Y.X. and B.Z. wrote the paper with input from all authors. X.H. coordinated and conceived the project.
Corresponding author
Ethics declarations
Competing interests
X.H., B.Z., Y.X., Q.Z., X.P. and T.Z. are inventors of a Chinese patent (application no. 2024117499275) covering the electroenzymatic method reported in this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–32, Supplementary Tables 1–21, Supplementary Methods, and additional references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, B., Xu, Y., Zhu, Q. et al. Electricity-driven enzymatic dynamic kinetic oxidation. Nature 643, 699–704 (2025). https://doi.org/10.1038/s41586-025-09178-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09178-6