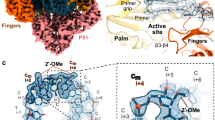
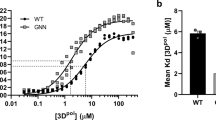
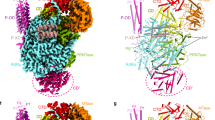
Abstract
Bacteria defend themselves from viral predation using diverse immune systems, many of which target foreign DNA for degradation1. Defence-associated reverse transcriptase (DRT) systems provide an intriguing counterpoint to this strategy by using DNA synthesis instead2,3. We and others recently showed that DRT2 systems use an RNA template to assemble a de novo gene that encodes the antiviral effector protein Neo4,5. It remains unclear whether similar mechanisms of defence are used by other related DRT families. Here, we show that DRT9 systems defend against phage using DNA homopolymer synthesis. Viral infection triggers polydeoxyadenylate (poly-dA) accumulation in the cell, driving abortive infection and population-level immunity. Cryo-electron microscopy structures reveal how a non-coding RNA serves as both a structural scaffold and reverse transcription template to direct hexameric complex assembly and poly-dA synthesis. Notably, biochemical and functional experiments identify tyrosine residues within the reverse transcriptase itself that probably prime DNA synthesis, leading to the formation of protein–DNA covalent adducts. Synthesis of poly-dA by DRT9 in vivo is regulated by the competing activities of phage-encoded triggers and host-encoded silencers. Collectively, our study identifies a nucleic-acid-driven defence system that expands the paradigm of bacterial immunity and broadens the known functions of reverse transcriptases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Next-generation sequencing data are available at the NCBI Gene Expression Omnibus (GEO) under accession numbers GSE295895, GSE295896 and GSE295897. The published genomes used for bioinformatics analyses were obtained from the NCBI (Supplementary Table 1). Cryo-EM maps and experimentally determined models were deposited at the appropriate public database (that is, EMDB, PDB, respectively) under accession numbers EMD-49525 and 9NLX (GST–DRT9 trimer), and EMD-49523 and 9NLV (DRT9 hexamer). Previously determined protein structures used for structural comparisons were obtained from the PDB (7R06 and 8FAK). The phylogenetic tree of UG RT homologues was obtained from ref. 3. MS data are available in the MassIVE database under accession number MSV000097825. All other data supporting the findings of this study are available in the Article and its Supplementary Information. Source data are provided with this paper.
Code availability
No custom scripts were developed for this Article.
References
Georjon, H. & Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 21, 686–700 (2023).
Gao, L. et al. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 369, 1077–1084 (2020).
Mestre, M. R. et al. UG/Abi: a highly diverse family of prokaryotic reverse transcriptases associated with defense functions. Nucleic Acids Res. 50, 6084–6101 (2022).
Tang, S. et al. De novo gene synthesis by an antiviral reverse transcriptase. Science 386, eadq0876 (2024).
Wilkinson, M. E., Li, D., Gao, A., Macrae, R. K. & Zhang, F. Phage-triggered reverse transcription assembles a toxic repetitive gene from a noncoding RNA. Science 386, eadq3977 (2024).
Duncan-Lowey, B. & Kranzusch, P. J. CBASS phage defense and evolution of antiviral nucleotide signaling. Curr. Opin. Immunol. 74, 156–163 (2022).
Bernheim, A. et al. Prokaryotic viperins produce diverse antiviral molecules. Nature 589, 120–124 (2021).
Tal, N. et al. Bacteria deplete deoxynucleotides to defend against bacteriophage infection. Nat. Microbiol. 7, 1200–1209 (2022).
Hsueh, B. Y. et al. Phage defence by deaminase-mediated depletion of deoxynucleotides in bacteria. Nat. Microbiol. 7, 1210–1220 (2022).
Silas, S. et al. Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase–Cas1 fusion protein. Science 351, aad4234 (2016).
Millman, A. et al. Bacterial retrons function in anti-phage defense. Cell 183, 1551–1561 (2020).
Bobonis, J. et al. Bacterial retrons encode phage-defending tripartite toxin–antitoxin systems. Nature 609, 144–150 (2022).
Carabias, A. et al. Retron-Eco1 assembles NAD+-hydrolyzing filaments that provide immunity against bacteriophages. Mol. Cell 84, 2185–2202 (2024).
Wang, C. et al. A reverse transcriptase-related protein mediates phage resistance and polymerizes untemplated DNA in vitro. Nucleic Acids Res. 39, 7620–7629 (2011).
Gapińska, M. et al. Structure-functional characterization of Lactococcus AbiA phage defense system. Nucleic Acids Res. 52, 4723–4738 (2024).
Figiel, M. et al. Mechanism of protein-primed template-independent DNA synthesis by Abi polymerases. Nucleic Acids Res. 50, 10026–10040 (2022).
González-Delgado, A., Mestre, M. R., Martínez-Abarca, F. & Toro, N. Prokaryotic reverse transcriptases: from retroelements to specialized defense systems. FEMS Microbiol. Rev. 45, fuab025 (2021).
Zoulim, F. & Seeger, C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J. Virol. 68, 6–13 (1994).
Yushenova, I. A. & Arkhipova, I. R. Biochemical properties of bacterial reverse transcriptase-related (rvt) gene products: multimerization, protein priming, and nucleotide preference. Curr. Genet. 64, 1287–1301 (2018).
Wang, J. et al. Complete genome sequence of bacteriophage T5. Virology 332, 45–65 (2005).
Glukhov, A. S., Krutilina, A. I., Kaliman, A. V., Shlyapnikov, M. G. & Ksenzenko, V. N. Bacteriophage T5 mutants carrying deletions in tRNA gene region. Mol. Biol. 52, 3–9 (2018).
Marians, K. J. PriA: at the crossroads of DNA replication and recombination. Prog. Nucleic Acid Res. Mol. Biol. 63, 39–67 (1999).
Sasaki, K. et al. Crystallization and preliminary crystallographic analysis of the N-terminal domain of PriA from Escherichia coli. Biochim. Biophys. Acta 1764, 157–160 (2006).
Rousset, F. et al. The impact of genetic diversity on gene essentiality within the Escherichia coli species. Nat. Microbiol. 6, 301–312 (2021).
Dillon, S. C. & Dorman, C. J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 8, 185–195 (2010).
Johansson, J. & Uhlin, B. E. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc. Natl Acad. Sci. USA 96, 10776–10781 (1999).
Srinivasan, R., Scolari, V. F., Lagomarsino, M. C. & Seshasayee, A. S. N. The genome-scale interplay amongst xenogene silencing, stress response and chromosome architecture in Escherichia coli. Nucleic Acids Res. 43, 295–308 (2015).
Burroughs, A. M., Zhang, D., Schäffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 43, 10633–10654 (2015).
Tan, J. M. J. et al. A DNA-gated molecular guard controls bacterial Hailong anti-phage defence. Nature https://doi.org/10.1038/s41586-025-09058-z (2025).
Tak, U., Walth, P. & Whiteley, A. T. Bacterial cGAS-like enzymes produce 2′,3′-cGAMP to activate an ion channel that restricts phage replication. Preprint at bioRxiv https://doi.org/10.1101/2023.07.24.550367 (2023).
Martin, G., Doublié, S. & Keller, W. Determinants of substrate specificity in RNA-dependent nucleotidyl transferases. Biochim. Biophys. Acta 1779, 206–216 (2008).
Motea, E. A. & Berdis, A. J. Terminal deoxynucleotidyl transferase: the story of a misguided DNA polymerase. Biochim. Biophys. Acta 1804, 1151–1166 (2010).
Lai, C. K., Miller, M. C. & Collins, K. Roles for RNA in telomerase nucleotide and repeat addition processivity. Mol. Cell 11, 1673–1683 (2003).
Baba, T. et al. Construction of Escherichia coli K‐12 in‐frame, single‐gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Cantalapiedra, C. P., Hernández-Plaza, A., Letunic, I., Bork, P. & Huerta-Cepas, J. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38, 5825–5829 (2021).
Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Nawrocki, E. P. & Eddy, S. R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29, 2933–2935 (2013).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. Publ. Protein Soc. 27, 14–25 (2018).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.Journal 17, 10–12 (2011).
Vasimuddin, M., Misra, S., Li, H. & Aluru, S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In Proc 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS) 314–324 (IEEE, 2019).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Blue, S. M. et al. Transcriptome-wide identification of RNA-binding protein binding sites using seCLIP-seq. Nat. Protoc. 17, 1223–1265 (2022).
Bailey, T. L., Johnson, J., Grant, C. E. & Noble, W. S. The MEME suite. Nucleic Acids Res. 43, W39–W49 (2015).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Will, S., Joshi, T., Hofacker, I. L., Stadler, P. F. & Backofen, R. LocARNA-P: accurate boundary prediction and improved detection of structural RNAs. RNA 18, 900–914 (2012).
Rivas, E., Clements, J. & Eddy, S. R. A statistical test for conserved RNA structure shows lack of evidence for structure in lncRNAs. Nat. Methods 14, 45–48 (2017).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Bouvette, J. et al. Automated systematic evaluation of cryo-EM specimens with SmartScope. eLife 11, e80047 (2022).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Cui, Y. et al. BioCircos.js: an interactive Circos JavaScript library for biological data visualization on web applications. Bioinformatics 32, 1740–1742 (2016).
Liscovitch-Brauer, N. et al. Profiling the genetic determinants of chromatin accessibility with scalable single-cell CRISPR screens. Nat. Biotechnol. 39, 1270–1277 (2021).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188 (2014).
Hughes, C. S. et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 14, 68–85 (2019).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Gilchrist, C. L. M., Mirdita, M. & Steinegger, M. Multiple protein structure alignment at scale with FoldMason. Preprint at bioRxiv https://doi.org/10.1101/2024.08.01.606130 (2024).
Acknowledgements
We thank A. I. Palmieri, S. Kang and A. J. Robinson for laboratory support; P. Sims for discussions regarding sequencing data analysis; S. Tavazoie, M. Liu and P. Oikonomou for sharing Keio knockout strains; D. Puleston and R. Sorek for metabolomics support; L. E. Berchowitz for Southern blotting support; the members of the JP Sulzberger Columbia Genome Center for next-generation sequencing support; and L. F. Landweber for qPCR and gel imager instrument access. Research in the Wiedenheft laboratory is supported by the National Institutes of Health (NIH R35GM134867), the M.J. Murdock Charitable Trust and the Montana Agricultural Experimental Station. Funding for the Montana State University Cryo-EM Core Facility (RRID: SCR_026324) was contributed by the National Science Foundation (DBI-1828765), the M.J. Murdock Charitable Trust, NIGMS (P30GM140963) and the MSU Office of Research, Economic Development and Graduate Education. Image processing was performed using the Tempest High Performance Computing System, operated and supported by University Information Technology Research Cyberinfrastructure (RRID: SCR_026229) at Montana State University. S.T. was supported by a Ruth L. Kirchstein Individual Predoctoral Fellowship (F30AI183830) from the NIH; N.B. by a Ruth L. Kirschstein Individual Predoctoral Fellowship (GM153146) from the NIH and received support from Montana INBRE (P20GM103474); L.M.K. by an NIH Medical Scientist Training Program grant (T32GM145440); M.J. by NIH/NIGMS grant R35GM152258; S.H.S. by NSF Faculty Early Career Development Program (CAREER) Award 2239685, a Pew Biomedical Scholarship, an Irma T. Hirschl Career Scientist Award, a Mallinckrodt Scholarship, the Howard Hughes Medical Institute Investigator Program and a generous startup package from the Columbia University Irving Medical Center Dean’s Office and the Vagelos Precision Medicine Fund.
Author information
Authors and Affiliations
Contributions
S.T., R.Ž. and S.H.S. conceived the project. S.T. and R.Ž. performed RIP–seq and cDIP–seq experiments, Southern blotting experiments and phage genetics experiments. S.T. and M.B. performed metabolomics experiments. S.T. and Y.M. performed proteomics experiments, with supervision from M.J. R.Ž. performed nucleoside-supplementation and oligo-spike-in experiments. N.B. prepared cryo-EM samples, collected and processed cryo-EM data, and built atomic models. S.P. purified SenDRT9-encoded RT–ncRNA complexes and performed all biochemical experiments, with assistance from R.A.W. J.L.R. performed plaque assays with ncRNA mutants, performed phage trigger and host factor experiments, and cloned and tested gp58 variants. L.M.K. cloned and screened DRT9 homologues for activity, isolated escaper phages, and performed and analysed phage whole-genome sequencing. T.W. performed phylogenetic and bioinformatics analyses and assisted in data interpretation and visualization. D.J.Z. constructed the ncRNA covariance model and performed growth curve experiments. G.D.L. assisted in the design and execution of phage whole-genome sequencing. S.T., R.Ž., N.B., S.P., J.L.R., L.M.K., T.W., B.W. and S.H.S. discussed the data and wrote the manuscript, with input from all of the authors.
Corresponding authors
Ethics declarations
Competing interests
S.H.S. is a co-founder and scientific advisor to Dahlia Biosciences, a scientific advisor to CrisprBits and Prime Medicine, and an equity holder in Dahlia Biosciences and CrisprBits. B.W. is the founder of SurGene and is listed as an inventor on patent applications related to CRISPR–Cas systems and applications thereof. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jeff Miller and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Screening and identification of active DRT9 immune systems.
a, Phylogenetic tree of DRT9-encoded RT homologues. Outer rings show SLATT protein and ncRNA association within nearby genomic neighbourhoods, and the inner ring shows previously identified DRT9 (UG28) homologues3; systems selected for experimental testing are indicated with red circles. b, Heatmap of pairwise amino acid sequence identity percentages among DRT9-encoded RT homologues tested in this study (left), and heatmap of phage defence activity for the same DRT9 systems tested against 10 diverse E. coli phages (right); RT proteins encoded N-terminal FLAG tags. c, Representative plaque assays demonstrating that SenDRT9 and PsaDRT9 exhibit broad defence against phages from the Tequatrovirus and Tequintavirus genera, as compared to an empty vector (EV) control. d, Plaque assays demonstrating that defence activity against T2 phage is completely abolished for both SenDRT9 and PsaDRT9 systems encoding catalytically inactive RT mutants (MUT). e, Growth curves of cells expressing WT or MUT SenDRT9 +/– T5 phage at the indicated multiplicity of infection (MOI). Data are shown as mean ± s.d. for n = 3 independent biological replicates. f, Plaque forming unit (PFU; top) and colony forming unit (CFU; bottom) measurements from an infection time course experiment in which cells expressing WT or MUT SenDRT9 were infected with T5 phage at a high MOI. Cells expressing the WT defence system attenuated phage replication but were unable to recover from infection, indicating that defence activation leads to abortive infection and cell death. Data are shown as mean ± s.d. for n = 3 independent biological replicates. g, Plaque assays demonstrating that SenDRT9 encoding an RT with an N-terminal FLAG, but not C-terminal FLAG, retains WT defence against T5 phage. h, AlphaFold 3 structure prediction of the RT monomer from SenDRT9, highlighting the predicted positions of the N- and C-termini (orange spheres) and YADD active site (red spheres).
Extended Data Fig. 2 Discovery and characterization of homopolymeric DNA products elicited by DRT9 immune systems.
a, Unmapped reads from a cDIP-seq dataset of SenDRT9-expressing cells infected with T5 phage, showing uninterrupted strings of poly-dT and poly-dA. b, RIP-seq and cDIP-seq coverage tracks (top to bottom) for either WT or RT-inactive (MUT) PsaDRT9, in the presence of T5 phage infection. A schematic of the genomic locus is shown below; data are normalized for sequencing depth and plotted as counts per million reads (CPM). c, MEME analysis results revealing a poly-dT motif enriched in unmapped reads from the WT + T5 cDIP-seq dataset in b. E, E-value significance; n, number of contributing sites. d, Methylene blue-stained membrane used for the Southern blot shown in Fig. 1h. Representative data are shown for experiments repeated at least two times with similar results. e, Schematic of oligo spike-in experiment to address potential bias in next-generation sequencing-based detection of poly-dA and poly-dT on the AVITI platform; P, phosphorylated 5′ end. f, Bar graph of dA25 and dT25 counts from total DNA sequencing of oligo spike-in experiments schematized in e; the apparent bias against poly-dA capture and sequencing leads to artificially elevated levels of dT25-containing reads relative to dA25-containing reads. g, Bar graph of normalized homopolymer counts from total RNA-seq datasets of WT and MUT SenDRT9-expressing cells +/– T5 phage infection. These data demonstrate that poly-dA and poly-dT cDNAs are not transcribed, in contrast to the cDNA products of DRT2. Data in f,g are shown as mean ± s.d. for n = 3 independent biological replicates.
Extended Data Fig. 3 Additional characterization of ncRNA sequence perturbations on SenDRT9 defence activity.
a, Covariance model of the DRT9 ncRNA from an analysis of 201 homologous systems (left), and WebLogo from a multiple sequence alignment centered around the putative U-rich template region (top right). b, Comparison of SenDRT9 (left) and KpnDRT2 (right) ncRNAs, highlighting the scaffold (grey) and template (orange) regions. Both ncRNAs template cDNA synthesis from a similar location relative to SL2 (reverse transcription start site, in red), and program repetitive cDNA synthesis across the template region. c, Representative plaque assays for the data shown in Fig. 2b. d, Heat map quantifying SenDRT9 defence activity against T5 phage for the indicated ncRNA mutations and deletions within the 3′-proximal SL6 and U-rich region, quantified as the fold reduction in EOP relative to an empty vector (EV) control; data are shown as the mean of n = 2 technical replicates. e, RIP-seq coverage tracks for SenDRT9 with WT ncRNA or the indicated ncRNA mutations in uninfected cells; the bottom three variants are mutated in the putative template region (residues 123–126). A schematic of the genomic locus is shown below; data are normalized for sequencing depth and plotted as counts per million reads (CPM). f, Heat map quantifying SenDRT9 defence activity against T2 phage for the same ncRNA mutations tested against T5 phage in Fig. 2b–d and panel d; data are shown as in d. g, Methylene blue-stained membrane used for the Southern blot shown in Fig. 2f. Representative data are shown for experiments repeated at least two times with similar results.
Extended Data Fig. 4 Purification and characterization of the SenDRT9-encoded RT-ncRNA complex.
a, E. coli expression vector design for the SenDRT9-encoded ncRNA and His6-GST-tagged RT. b, Size-exclusion chromatogram of the His6-GST-tagged RT-ncRNA complex separated on a Superdex 200 10/300 column (left), and SDS-PAGE analysis of the void volume and labelled peaks (right); the high A260:A280 ratio is consistent with a protein-nucleic acid complex. c, Denaturing, SYBR Gold-stained 10% urea-PAGE analysis of the ~150-nt ncRNA species co-purifying with SenRT after RNase or DNase treatment (top), and RNA-seq analysis of the ncRNA (middle). The mature ncRNA carries an extraneous 5′-G resulting from the T7 promoter and lacks SL6 at the 3′ end (bottom), suggesting that SL6 may be involved in transcriptional termination in vivo while being dispensable for RT-mediated poly-dA synthesis. d, Plaque assay showing loss of SenDRT9 defence activity for a His6-GST-tagged RT variant against T5 phage (left), but not T2 phage (right); EV, empty vector. e, Overlaid chromatograms from gel filtration experiments with RT-ncRNA complexes before and after TEV protease treatment of the His6-GST-RT fusion protein, revealing a shift to earlier retention volume and thus increased oligomeric state; the persistent high A260:A280 ratio suggests that the RT-ncRNA interaction remains intact. f, Denaturing 8% urea-PAGE analysis of DNA polymerization assays that contained 150 nM RT-ncRNA complex and increasing amounts of dATP per RT monomer, as indicated. Reactions were incubated at 37 °C for 60 min, followed by proteinase K treatment and phenol-chloroform extraction prior to electrophoretic separation; the gel was stained with SYBR Gold. For b,c,f, representative data are shown for experiments repeated at least two times with similar results.
Extended Data Fig. 5 Roles of C-terminal tyrosine residues in protein-primed reverse transcription and DRT9 phage defence.
a, Southern blot analysis of total DNA isolated from T5-infected cells expressing WT SenDRT9 +/– proteinase K treatment. The blot was probed with oligo-dT40 (left) to detect poly-dA species; the methylene blue-stained membrane after transfer is shown at right; M denotes a ladder marker. b, Overlaid chromatograms from gel filtration analysis of RT-ncRNA complexes +/– dATP incubation, revealing an increased A260:A280 ratio and a dramatic shift in retention volume in the presence of dATP. c, Volcano plot of differential peptide abundance from mass spectrometry analysis of WT versus catalytically inactive (MUT) RT proteins immunoprecipitated from T5-infected cells expressing SenDRT9. Each dot indicates a SenRT peptide, and blue dots indicate significantly depleted peptides with log2(fold change) ≤ 1.5 and adjusted p-value < 0.05, as determined by unpaired two-tailed t-test with correction for multiple comparisons using the Benjamini-Hochberg method; the most depleted peptide, IMNPQSLNYDYE, contains two tyrosine residues likely involved in priming of poly-dA synthesis. d, Plaque assay showing loss of SenDRT9 defence activity against T2 phage for a double Y496F,Y498F mutant, but not single Y496F or Y498F mutants; EV, empty vector. e, RIP-seq coverage tracks for SenDRT9 in T5-infected cells with WT and single or double Y496F/Y498F RT mutants, as indicated. A schematic of the genomic locus is shown below; data are normalized for sequencing depth and plotted as counts per million reads (CPM). f, Denaturing 5% urea-PAGE analysis of DNA polymerization assays with WT (left) and Y496F,Y498F mutant (right) RT. All reactions contained 20 nM RT-ncRNA and 100 µM [α-32P]-dATP; M denotes a DNA ladder marker. g, Southern blot analysis of total DNA isolated from T5-infected cells expressing SenDRT9 with WT and single or double Y496F/Y498F RT mutants, as indicated. The blot was probed with oligo-dT40 (left) to detect poly-dA species; the methylene blue-stained membrane after transfer is shown at right; M denotes a DNA ladder marker. h, SDS-PAGE analysis of DNA polymerization assays with either WT SenDRT9 or a U4 > A4 mutant ncRNA. RT-ncRNA complexes (0.15 µM) were incubated with the indicated dNTPs (0.9 mM). The red arrow denotes high-molecular weight (MW) protein-DNA conjugates; the SUMO-RT band arises from incomplete tag removal; *, purification contaminant; M, protein ladder marker. i, SDS-PAGE analysis of DNA polymerization assays as in h, but with a U4 > G4 mutant ncRNA; no high-MW species are observed. j, Denaturing, SYBR Gold-stained 10% urea-PAGE analysis (left) of WT, U4 > A4, and U4 > G4 ncRNAs co-purifying with SenRT. The absence of a band for the U4 > C4 ncRNA, despite a clear band corresponding to the RT by SDS-PAGE (right), indicates that the U4 > C4 mutation disrupts RT-ncRNA complex formation. For a,f-j, representative data are shown for experiments repeated at least two times with similar results.
Extended Data Fig. 6 Cryo-EM analysis and image processing workflow for trimeric SenDRT9 RT-ncRNA complex.
a, Representative micrograph from SenDRT9 RT-ncRNA complex data collection with the His6-GST fusion protein, revealing a densely-packed field of particles in vitreous ice. b, Initial consensus volume after processing blob-picked particles, which refined to 3.7 Å resolution (C3-reconstruction) for de novo template generation and subsequent particle picking. c, Representative selected 2D classes from 5,235,086 initial particles picked using the template in panel b. d, Four-class ab initio reconstruction from 2,530,970 particles in selected classes to remove junk particles. e, Results of a five-class 3D classification without a mask and filtered at 8 Å resolution, with a class similarity of 0.1 to remove remaining poorly aligned particles. The best volume, highlighted by a green background, contained 367,640 particles that were selected for further processing. f, View of the final reconstruction in g shown at high contour (top), revealing noisy density at the N-terminus of each RT monomer corresponding to the His6-GST fusion used for expression and purification. We hypothesized that the GST fusion would prevent the formation of higher-order oligomers (bottom). g, Final (C3) reconstruction that refined to 3 Å resolution and was used for building the trimeric RT-ncRNA complex model. The map is shown with partial transparency and coloured according to the model, with RT subunits in blue and ncRNA subunits in orange. h, Plot of Cryosparc’s gold standard Fourier shell correlation for the map in g. i, Plot of particle view distribution reveals mild anisotropy but no missing views. j, SDS-PAGE analysis of DNA polymerization assays with His6-GST-RT sample, in which RT-ncRNA complexes were incubated with the indicated dNTP(s) before reactions were quenched and resolved electrophoretically. Red arrows indicate preliminary evidence of protein-DNA conjugates in reactions that contained dATP. Representative data are shown for experiments repeated at least two times with similar results.
Extended Data Fig. 7 Cryo-EM analysis and image processing workflow for hexameric SenDRT9 RT-ncRNA complex.
a, Representative micrograph from the SenDRT9 RT-ncRNA hexameric complex, revealing a monolayer of evenly distributed particles. b, Initial reconstruction from on-the-fly image analysis that was used to generate de novo templates used for particle picking. c, Representative 2D Classes from 6,011,113 template-picked particles. d, Initial volumes from a 7-class ab initio reconstruction of 1,411,369 particles. Selected volumes for downstream processing are shown with a green background. e, Final steps used to sort selected particles in d to yield the final reconstruction. f, Final (D3) reconstruction of the SenDRT9 RT-ncRNA hexamer that refined to 2.6 Å and was used for model building. The map is shown as a partially transparent surface, with RT subunits shown in blue/cyan and ncRNA subunits shown in orange/salmon. g, Plot of Cryosparc’s gold standard Fourier shell correlation for the map in f. h, Plot of particle view distribution reveals mild anisotropy but no missing views.
Extended Data Fig. 8 Additional features of the SenDRT9 hexamer visualized by cryo-EM.
a, Local resolution map of the SenDRT9 RT-ncRNA hexamer (FSC cutoff 0.143), showing that resolution falls off near the periphery of the complex in flexible regions such as SL3 and SL4. b, Semi-transparent density highlighting a network of protein-RNA interactions that stabilize SL5. c, Semi-transparent density highlighting protein-protein interactions that stabilize the back-to-back assembly of trimers. d, Difference map generated by subtracting a calculated map of the model from the experimental density. Unmodelled density (grey) is visible in the map directly adjacent to the YADD active site (red), which may correspond to a small stretch of poly-dA product; repeated efforts to sort these particles failed to separate distinct conformational states. e, A close-up view of the SenRT active site (YADD residues in red). Density for the RT is shown as a semi-transparent surface, and the density map for the ncRNA is omitted here for simplicity. f, Semi-transparent density map highlighting the ncRNA poly-G track (residues 80-86) that was used to establish register for ModelAngelo.
Extended Data Fig. 9 Activation of DRT9 immunity by phage-encoded factors.
a, Representative plaque assays with WT or escaper T5 phage variants tested in strains expressing either the WT or RT-inactive (MUT) SenDRT9 system. b, Bar graphs of relative nucleotide levels for the indicated species quantified by LC-MS/MS, in lysates from cells expressing SenDRT9 or an empty vector (EV) control +/– T5 phage infection. Data are shown as the mean for n = 2 independent biological replicates. c, Bar graphs of plaque forming units per mL (PFU/mL) of T5 phage lysates after liquid culture infection of cells expressing SenDRT9 or an EV control, in the presence of the indicated supplemented nucleosides; data are shown as the mean for n = 2 independent biological replicates. d, Methylene blue-stained membrane of total DNA from cells expressing WT or MUT SenDRT9 +/– gp58 induction, for the Southern blot shown in Fig. 5f. e, Western blot analysis of SenRT co-immunoprecipitation with gp58. Immunoprecipitation was performed with a FLAG antibody on lysates from cells co-expressing SenRT (WT or MUT, FLAG-tagged or untagged) with V5-tagged gp58, followed by Western blot analysis using a V5 antibody. The unique presence of a band corresponding to gp58 in the IP eluate from cells expressing WT SenDRT9 indicates a DNA-dependent interaction between the RT and gp58. f, Denaturing 5% urea-PAGE analysis of DNA polymerization assays, after reactions were treated with ExoI (left) or Nuclease P1 (right) in the presence of increasing concentrations of T5 gp58. All reactions contained 20 nM RT-ncRNA (U4 > A4) and 100 µM [α-32P]-dTTP and were incubated at 37 °C for 60 min prior to addition of gp58 and ExoI or Nuclease P1. For d-f, representative data are shown for experiments repeated at least two times with similar results.
Supplementary information
Supplementary Figs. 1 and 2
Supplementary Fig. 1: uncropped images of electrophoretic separation assays and LB agar culture plates. Supplementary Fig. 2: comparison of domain composition and 3D structure across evolutionarily diverse RT homologues.
Supplementary Tables 1–8
Supplementary Table 1: DRT9-encoded RT homologues in the Extended Data Fig. 1a phylogenetic tree. Supplementary Table 2: list of DRT9-family immune systems tested in this study. Supplementary Table 3: genotypes of escaper phages that evade DRT9 immunity. Supplementary Table 4: strains used in this study. Supplementary Table 5: description and sequence of plasmids used in this study. Supplementary Table 6: probes and oligonucleotides used in this study. Supplementary Table 7: conditions of MRM transitions from metabolomics measurements. Supplementary Table 8: IP–MS hits plotted in Fig. 6d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, S., Žedaveinytė, R., Burman, N. et al. Protein-primed homopolymer synthesis by an antiviral reverse transcriptase. Nature 643, 1352–1362 (2025). https://doi.org/10.1038/s41586-025-09179-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09179-5