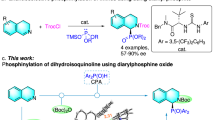
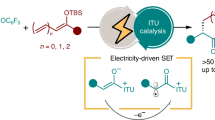
Abstract
The synthesis of enantiopure compounds is a central focus in organic chemistry owing to the prevalence of chiral centres in biological systems and the impact of homochirality on molecular properties. With growing recognition of electrochemistry as a powerful tool to improve the scope and sustainability of organic synthesis1, increasing efforts have been directed towards developing asymmetric electrocatalytic reactions to access challenging chiral molecules2,3,4. However, many useful electrochemical reactions rely on direct electrolysis without a catalyst, making them inherently difficult to render enantioselective. Supporting electrolytes are integral to electrochemical systems and, in addition to ensuring sufficient solution conductivity, they can influence the rate and selectivity of electrochemical transformations5. Chiral supporting electrolytes can mediate asymmetric reactions via direct electrolysis, but their use in organic electrosynthesis remains largely unexplored6,7. Here we describe the use of substoichiometric chiral phosphate salts as supporting electrolytes to facilitate the oxidation of racemic trivalent phosphines to afford enantioenriched phosphine oxides. Our approach relies on a dynamic-kinetic-resolution strategy that exploits the rapid pyramidal inversion of an anodically generated phosphoniumyl radical cation8, while a high concentration of chiral phosphate at the electrode–electrolyte interface9,10 enhances enantioselective control during rate-limiting nucleophilic addition. Our results highlight the promise of chiral supporting electrolytes for promoting radical-ion-mediated asymmetric transformations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this work are available in the paper and its Supplementary Information. Full X-ray structural data are available free of charge from the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers 2385247 and 2385248.
References
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Lin, Q., Li, L. & Luo, S. Asymmetric electrochemical catalysis. Chem. Eur. J. 25, 10033–10044 (2019).
Jiao, K.-J. et al. The applications of electrochemical synthesis in asymmetric catalysis. Chem Catal. 2, 3019–3047 (2022).
Rein, J., Zacate, S. B., Mao, K. & Lin, S. A tutorial on asymmetric electrocatalysis. Chem. Soc. Rev. 52, 8106–8125 (2023).
Moeller, K. Anodic olefin coupling reactions: a mechanism driven approach to the development of new synthetic tools. Electrochem. Soc. Interface 25, 53–59 (2016).
Maekawa, H., Itoh, K., Goda, S. & Nishiguchi, I. Enantioselective electrochemical oxidation of enol acetates using a chiral supporting electrolyte. Chirality 15, 95–100 (2003).
Yadav, A. K., Manju, M. & Chhinpa, P. R. Enantioselective cathodic reduction of some prochiral ketones in the presence of (−)-N,N′-dimethylquininium tetrafluoroborate at mercury cathode. Tetrahedron Asymmetry 14, 1079–1081 (2003).
Reichl, K. D., Ess, D. H. & Radosevich, A. T. Catalyzing pyramidal inversion: configurational lability of P-stereogenic phosphines via single electron oxidation. J. Am. Chem. Soc. 135, 9354–9357 (2013).
Bard, A. & Faulkner, L. R. (eds) in Electrochemical Methods Fundamentals and Applications 534–579 (John Wiley, 2001).
Feng, G., Huang, J., Sumpter, B. G., Meunier, V. & Qiao, R. Structure and dynamics of electrical double layers in organic electrolytes. Phys. Chem. Chem. Phys. 12, 5468–5479 (2010).
von Münchow, T., Dana, S., Xu, Y., Yuan, B. & Ackermann, L. Enantioselective electrochemical cobalt-catalyzed aryl C–H activation reactions. Science 379, 1036–1042 (2023).
Song, L. et al. Dual electrocatalysis enables enantioselective hydrocyanation of conjugated alkenes. Nat. Chem. 12, 747–754 (2020).
Wang, Z.-H. et al. TEMPO-enabled electrochemical enantioselective oxidative coupling of secondary acyclic amines with ketones. J. Am. Chem. Soc. 143, 15599–15605 (2021).
Naulin, E. et al. Stereoselective synthesis of fissoldhimine alkaloid analogues via sequential electrooxidation and heterodimerization of ureas. Chem. Commun. 60, 11560–11563 (2024).
Komori, T. & Nonaka, T. Stereochemical studies of the electrolytic reactions of organic compounds. 25. Electroorganic reactions on organic electrodes. 6. Electrochemical asymmetric oxidation of unsymmetric sulfides to the corresponding chiral sulfoxides on poly(amino acid)-coated electrodes. J. Am. Chem. Soc. 106, 2656–2659 (1984).
Reidell, A. C., Pazder, K. E., LeBarron, C. T., Stewart, S. A. & Hosseini, S. Modified working electrodes for organic electrosynthesis. ACS Org. Inorg. Au 4, 579–603 (2024).
Schmittel, M. & Burghart, A. Understanding reactivity patterns of radical cations. Angew. Chem. Int. Ed. 36, 2550–2589 (1997).
Gentry, E. C., Rono, L. J., Hale, M. E., Matsuura, R. & Knowles, R. R. Enantioselective synthesis of pyrroloindolines via noncovalent stabilization of indole radical cations and applications to the synthesis of alkaloid natural products. J. Am. Chem. Soc. 140, 3394–3402 (2018).
Das, S. et al. Asymmetric counteranion-directed photoredox catalysis. Science 379, 494–499 (2023).
Ohmura, S. et al. Highly enantioselective radical cation [2 + 2] and [4 + 2] cycloadditions by chiral iron(III) photoredox catalysis. J. Am. Chem. Soc. 145, 15054–15060 (2023).
Xu, Z. et al. Asymmetric counteranion‐directed electrocatalysis for enantioselective control of radical cation. Angew. Chem. Int. Ed. 64, e202413601 (2024).
Guo, H., Fan, Y. C., Sun, Z., Wu, Y. & Kwon, O. Phosphine organocatalysis. Chem. Rev. 118, 10049–10293 (2018).
Imamoto, T. P-stereogenic phosphorus ligands in asymmetric catalysis. Chem. Rev. 124, 8657–8739 (2024).
Dutartre, M., Bayardon, J. & Jugé, S. Applications and stereoselective syntheses of P-chirogenic phosphorus compounds. Chem. Soc. Rev. 45, 5771–5794 (2016).
Montchamp, J.-L. Phosphorus Chemistry I: Asymmetric Synthesis and Bioactive Compounds (Springer, 2015).
Bergin, E. et al. Synthesis of P-stereogenic phosphorus compounds. Asymmetric oxidation of phosphines under Appel conditions. J. Am. Chem. Soc. 129, 9566–9567 (2007).
Rajendran, K. V., Kennedy, L. & Gilheany, D. G. P-stereogenic phosphorus compounds: effect of aryl substituents on the oxidation of arylmethylphenylphosphanes under asymmetric Appel conditions. Eur. J. Org. Chem. 2010, 5642–5649 (2010).
Perlikowska, W., Gouygou, M., Daran, J., Balavoineb, G. & Mikołajczyka, M. Kinetic resolution of P-chiral tertiary phosphines and chlorophosphines: a new approach to optically active phosphoryl and thiophosphoryl compounds. Tetrahedron Lett. 42, 7841–7845 (2001).
Rusmore, T. A., Behlen, M. J., John, A., Glatzhofer, D. T. & Nicholas, K. M. Oxidative kinetic resolution of P-chiral phosphines catalyzed by chiral (salen)dioxomolybdenum complexes. Mol. Catal. 513, 111776 (2021).
Jennings, E. V., Nikitin, K., Ortin, Y. & Gilheany, D. G. Degenerate nucleophilic substitution in phosphonium salts. J. Am. Chem. Soc. 136, 16217–16226 (2014).
Ohmori, H., Nakai, S., Sekiguchi, M. & Masui, M. Anodic oxidation of organophosphorus compounds. III. Anodic alkoxylation and thioalkoxylation of triphenylphosphine. Chem. Pharm. Bull. 28, 910–915 (1980).
Maeda, H., Koide, T., Maki, T. & Ohmori, H. Electrochemical preparation and some reactions of alkoxy triphenylphosphonium ions. Chem. Pharm. Bull. 43, 1076–1080 (1995).
Maeda, H., Maki, T., Eguchi, K., Koide, T. & Ohmori, H. One-step deoxygenation of alcohols into alkanes by a ‘double electrolysis’ in the presence of a phosphine. Tetrahedron Lett. 35, 4129–4132 (1994).
Sakai, K., Nagai, N., Mizuki, Y., Masui, M. & Ohmori, H. Reaction of electrochemically generated triphenylphosphine radical cation with amides and ureas. Chem. Pharm. Bull. 33, 373–376 (1985).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013).
Imamoto, T., Kikuchi, S. I., Miura, T. & Wada, Y. Stereospecific reduction of phosphine oxides to phosphines by the use of a methylation reagent and lithium aluminum hydride. Org. Lett. 3, 87–90 (2001).
Rajendran, K. V. & Gilheany, D. G. Simple unprecedented conversion of phosphine oxides and sulfides to phosphine boranes using sodium borohydride. Chem. Commun. 48, 817–819 (2012).
Wang, T., Han, X., Zhong, F., Yao, W. & Lu, Y. Amino acid-derived bifunctional phosphines for enantioselective transformations. Acc. Chem. Res. 49, 1369–1378 (2016).
Greenfield, S. J., Agarkov, A. & Gilbertson, S. R. High asymmetric induction with β-turn-derived palladium phosphine complexes. Org. Lett. 5, 3069–3072 (2003).
Mino, T., Kashiharab, K. & Yamashita, M. New chiral phosphine–amide ligands in palladium-catalysed asymmetric allylic alkylations. Tetrahedron Asymmetry 12, 287–291 (2001).
Kütt, A. Strengths of acids in acetonitrile. Eur. J. Org. Chem. 2021, 1407–1419 (2021).
Warner, C. J. A., Reeder, A. T. & Jones, S. P-chiral phosphine oxide catalysed reduction of prochiral ketimines using trichlorosilane. Tetrahedron Asymmetry 27, 136–141 (2016).
Laudadio, G. et al. Sulfonamide synthesis through electrochemical oxidative coupling of amines and thiols. J. Am. Chem. Soc. 141, 5664–5668 (2019).
Rajendran, K. V. & Gilheany, D. G. Identification of a key intermediate in the asymmetric Appel process: one pot stereoselective synthesis of P-stereogenic phosphines and phosphine boranes from racemic phosphine oxides. Chem. Commun. 48, 10040–10042 (2012).
Guo, X., Price, N. G. & Zhu, Q. Electrochemical cyanation of alcohols enabled by an iodide-mediated phosphine P(V/III) redox couple. Org. Lett. 26, 7347–7351 (2024).
Zhang, J., Mück-Lichtenfeld, C. & Studer, A. Photocatalytic phosphine-mediated water activation for radical hydrogenation. Nature 619, 506–513 (2023).
Xie, Z.-Z. et al. Photoredox-catalyzed selective α-scission of PR3–OH radicals to access hydroalkylation of alkenes. Org. Lett. 25, 9014–9019 (2023).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Creary, X., Mehrsheikh-Mohammadi, M. E. & Mcdonald, S. Methylenecyclopropane rearrangement as a probe for free radical substituent effects. σ· values for commonly encountered conjugating and organometallic groups. J. Org. Chem. 52, 3254–3263 (1987).
Sharma, S. Electro-organic reactions: direct and indirect electrolysis. Orient. J. Chem. 40, 321–332 (2024).
Lu, T. & Chen, Q. Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J. Comput. Chem. 43, 539–555 (2022).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024).
Pracht, P. et al. CREST—a program for the exploration of low-energy molecular chemical space. J. Chem. Phys. 160, 114110 (2024).
Lu, T. Molclus program, version 1.12 (Beijing Kein Research Center for Natural Sciences, 2023); http://www.keinsci.com/research/molclus.html.
Acknowledgements
This work was supported by NSF Center for Synthetic Organic Electrochemistry (CHE-2002158) and Bristol Myers Squibb Graduate Fellowship (K.M.). S.L. is grateful for a Camille Dreyfus Teacher-Scholar Award. We thank L. Novaes for initial contribution to the development of the thioether oxidation; I. Crooker for assistance in preparing supporting electrolytes and substrates; S. MacMillan for collecting and elucidating X-ray crystallography data; I. Keresztes for discussion on NMR structural analysis; A. L. Lai (funded by NIH R24GM146107 and R35GM148272) for help with electron paramagnetic resonance experiments; B. Gorski for reproducing the reaction; D. Toste for discussions on chiral phosphoric acid synthesis and catalysis; G. Laudadio for discussions on flow electrochemistry; Y. Yang for discussions on the electrode–electrolyte interface and electrical double layer; D. B. Collum for providing access to his computational resources; K. R. Meihaus for manuscript editing; and W.-C. C. Lee for figure editing.
Author information
Authors and Affiliations
Contributions
S.L. and Y.Q. supervised the project. S.L., C.L. and K.M. conceived of the project. C.L., K.M. and C.M. performed synthetic experiments. K.M. and C.L. performed experimental mechanistic studies. Y.W. performed DFT calculations. C.G. performed molecular dynamics simulations. J.M.P. performed flow cell experiments. N.I.C. performed chemical space analysis. The paper was written by K.M., C.L., Y.W. and S.L., edited by C.G. and Y.Q., and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, K., Liu, C., Wang, Y. et al. Dynamic kinetic resolution of phosphines with chiral supporting electrolytes. Nature 643, 1288–1296 (2025). https://doi.org/10.1038/s41586-025-09238-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09238-x