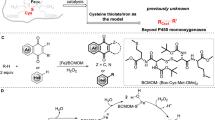
Abstract
Transition metal–hydrides have been widely exploited in catalysis for the hydrofunctionalization of unsaturated moieties, including carbonyls, alkenes and alkynes1. To complement heterolytic metal–hydride bond cleavage, metal–hydride hydrogen atom transfer (MHAT) has recently gained attention, as a promising strategy for radical hydrofunctionalization of unactivated alkenes2, thus enabling late-stage diversification of complex molecules3,4. However, owing to the weak interactions between the prochiral organic radical and the enantiopure catalyst5, asymmetric MHAT6 remains challenging. Here we show that cytochrome P450 enzymes (CYPs) can be repurposed to catalyse asymmetric MHAT, a new-to-nature reaction. Directed evolution of P450BM3 yielded a triple mutant that catalyses MHAT radical cyclization of unactivated alkenes, producing diverse cyclic compounds—including pyrrolidines and piperidines—with up to 98:2 enantiomeric ratio under aerobic whole-cell conditions. Apart from electron-deficient alkenes, alternative radical acceptors—including hydrazones, oximes and nitriles—were converted by repurposed P450BM3 to enantioenriched cyclization products. Mechanistic investigations support an MHAT mechanism proceeding by homolytic cleavage of a fleeting iron(III)–hydride species2,6. Starting from CYP119, directed evolution afforded a stereocomplementary MHATase, highlighting the potential of repurposed CYPs for MHAT biocatalysis. Our study highlights the prospect of integrating homolytic metal–hydride reactivity into metalloenzymes, thus expanding the scope of asymmetric radical biocatalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings in this study are available in this paper and the Supplementary Information. Source data are provided with this paper.
References
Maurizio Peruzzini, R. P. Recent Advances in Hydride Chemistry 1st edn (Elsevier Science, 2002).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Wu, J. & Ma, Z. Metal-hydride hydrogen atom transfer (MHAT) reactions in natural product synthesis. Org. Chem. Front. 8, 7050–7076 (2021).
Green, S. A. et al. The high chemofidelity of metal-catalyzed hydrogen atom transfer. Acc. Chem. Res. 51, 2628–2640 (2018).
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Shevick, S. L. et al. Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals. Chem. Sci. 11, 12401–12422 (2020).
Schilter, D., Camara, J. M., Huynh, M. T., Hammes-Schiffer, S. & Rauchfuss, T. B. Hydrogenase enzymes and their synthetic models: the role of metal hydrides. Chem. Rev. 116, 8693–8749 (2016).
Lee, W.-C. C., Wang, D.-S., Zhu, Y. & Zhang, X. P. Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism. Nat. Chem. 15, 1569–1580 (2023).
Mukaiyama, T. et al. Oxidation-reduction hydration of olefins with molecular oxygen and 2-propanol catalyzed by bis(acetylacetonato)cobalt(II). Chem. Lett. 18, 449–452 (1989).
Waser, J. & Carreira, E. M. Convenient synthesis of alkylhydrazides by the cobalt-catalyzed hydrohydrazination reaction of olefins and azodicarboxylates. J. Am. Chem. Soc. 126, 5676–5677 (2004).
Choi, J., Tang, L. & Norton, J. R. Kinetics of hydrogen atom transfer from (η5-C5H5)Cr(CO)3H to various olefins: influence of olefin structure. J. Am. Chem. Soc. 129, 234–240 (2007).
Ishikawa, H. et al. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J. Am. Chem. Soc. 131, 4904–4916 (2009).
Ma, X. S. & Herzon, S. B. Intermolecular hydropyridylation of unactivated alkenes. J. Am. Chem. Soc. 138, 8718–8721 (2016).
Lo, J. C., Yabe, Y. & Baran, P. S. A practical and catalytic reductive olefin coupling. J. Am. Chem. Soc. 136, 1304–1307 (2014).
Discolo, C. A., Touney, E. E. & Pronin, S. V. Catalytic asymmetric radical-polar crossover hydroalkoxylation. J. Am. Chem. Soc. 141, 17527–17532 (2019).
Ebisawa, K. et al. Catalyst- and silane-controlled enantioselective hydrofunctionalization of alkenes by cobalt-catalyzed hydrogen atom transfer and radical-polar crossover. J. Am. Chem. Soc. 142, 13481–13490 (2020).
Zhang, G. & Zhang, Q. Cobalt-catalyzed HAT reaction for asymmetric hydrofunctionalization of alkenes and nucleophiles. Chem Catal. 3, 100526 (2023).
Wang, J.-J. et al. Mimicking hydrogen-atom-transfer-like reactivity in copper-catalysed olefin hydrofunctionalization. Nat. Catal. 7, 838–846 (2024).
Buzsaki, S. R. et al. Fe/thiol cooperative hydrogen atom transfer olefin hydrogenation: mechanistic insights that inform enantioselective catalysis. J. Am. Chem. Soc. 146, 17296–17310 (2024).
Hammer, S. C., Marjanovic, A., Dominicus, J. M., Nestl, B. M. & Hauer, B. Squalene hopene cyclases are protonases for stereoselective Brønsted acid catalysis. Nat. Chem. Biol. 11, 121–126 (2015).
Gergel, S. et al. Engineered cytochrome P450 for direct arylalkene-to-ketone oxidation via highly reactive carbocation intermediates. Nat. Catal. 6, 606–617 (2023).
Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339, 307–310 (2013).
Vargas, D. A. et al. Biocatalytic strategy for the construction of sp3-rich polycyclic compounds from directed evolution and computational modelling. Nat. Chem. 16, 817–826 (2024).
Bruffy, S. K. et al. Biocatalytic asymmetric aldol addition into unactivated ketones. Nat. Chem. 16, 2076–2083 (2024).
Nakano, Y., Biegasiewicz, K. F. & Hyster, T. K. Biocatalytic hydrogen atom transfer: an invigorating approach to free-radical reactions. Curr. Opin. Chem. Biol. 49, 16–24 (2019).
Van Stappen, C. et al. Designing artificial metalloenzymes by tuning of the environment beyond the primary coordination sphere. Chem. Rev. 122, 11974–12045 (2022).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Rui, J. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)-H azidation. Science 376, 869–874 (2022).
Zetzsche, L. E. et al. Biocatalytic oxidative cross-coupling reactions for biaryl bond formation. Nature 603, 79–85 (2022).
Xu, Y. et al. A light-driven enzymatic enantioselective radical acylation. Nature 625, 74–78 (2024).
Chen, R., Kayrouz, C. S., McAmis, E., Clark, D. S. & Hartwig, J. F. Carbonic anhydrase variants catalyze the reduction of dialkyl ketones with high enantioselectivity. Angew. Chem. Int. Ed. 63, e202407111 (2024).
Ji, P., Park, J., Gu, Y., Clark, D. S. & Hartwig, J. F. Abiotic reduction of ketones with silanes catalysed by carbonic anhydrase through an enzymatic zinc hydride. Nat. Chem. 13, 312–318 (2021).
Zhang, X. et al. Repurposing myoglobin into an abiological asymmetric ketoreductase. Chem 10, 2577–2589 (2024).
Wan, Z. et al. Stereoconvergent reduction of alkenes using a repurposed iron-based dioxygenase. Nat. Synth. https://doi.org/10.1038/s44160-025-00788-6 (2025).
Li, J., Kumar, A. & Lewis, J. C. Non-native intramolecular radical cyclization catalyzed by a B12-dependent enzyme. Angew. Chem. Int. Ed. 62, e202312893 (2023).
Wang, B. et al. Repurposing iron- and 2-oxoglutarate-dependent oxygenases to catalyze olefin hydration. Angew. Chem. Int. Ed. 62, e202311099 (2023).
Chen, D. et al. An evolved artificial radical cyclase enables the construction of bicyclic terpenoid scaffolds via an H-atom transfer pathway. Nat. Chem. 16, 1656–1664 (2024).
Fansher, D. J., Besna, J. N., Fendri, A. & Pelletier, J. N. Choose your own adventure: a comprehensive database of reactions catalyzed by cytochrome P450 BM3 variants. ACS Catal. 14, 5560–5592 (2024).
Kim, D., Rahaman, S. M. W., Mercado, B. Q., Poli, R. & Holland, P. L. Roles of iron complexes in catalytic radical alkene cross-coupling: a computational and mechanistic study. J. Am. Chem. Soc. 141, 7473–7485 (2019).
Li, H. Y., Darwish, K. & Poulos, T. L. Characterization of recombinant Bacillus megaterium cytochrome P-450 BM-3 and its two functional domains. J. Biol. Chem. 266, 11909–11914 (1991).
Gröger, H., Gallou, F. & Lipshutz, B. H. Where chemocatalysis meets biocatalysis: in water. Chem. Rev. 123, 5262–5296 (2023).
Andersson, M. P., Gallou, F., Klumphu, P., Takale, B. S. & Lipshutz, B. H. Structure of nanoparticles derived from designer surfactant TPGS-750-M in water, as used in organic synthesis. Chem. Eur. J. 24, 6778–6786 (2018).
Turner, O. J., Murphy, J. A., Hirst, D. J. & Talbot, E. P. A. Hydrogen atom transfer-mediated cyclisations of nitriles. Chem. Eur. J. 24, 18658–18662 (2018).
Saladrigas, M., Loren, G., Bonjoch, J. & Bradshaw, B. Hydrogen atom transfer (HAT)-triggered iron-catalyzed intra- and intermolecular coupling of alkenes with hydrazones: access to complex amines. ACS Catal. 8, 11699–11703 (2018).
Drienovská, I., Mayer, C., Dulson, C. & Roelfes, G. A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue. Nat. Chem. 10, 946–952 (2018).
Kato, S., Abe, M., Gröger, H. & Hayashi, T. Reconstitution of myoglobin with iron porphycene generates an artificial aldoxime dehydratase with expanded catalytic activities. ACS Catal. 14, 13081–13087 (2024).
Chen, K., Wang, Z., Ding, K., Chen, Y. & Asano, Y. Recent progress on discovery and research of aldoxime dehydratases. Green Synth. Catal. 2, 179–186 (2021).
Mohamed, H., Ghith, A. & Bell, S. G. The binding of nitrogen-donor ligands to the ferric and ferrous forms of cytochrome P450 enzymes. J. Inorg. Biochem. 242, 112168 (2023).
Omura, T. & Sato, R. The carbon monoxide-binding pigment of liver microsomes: I. Evidence for its hemoprotein nature. J. Biol. Chem. 239, 2370–2378 (1964).
Zheng, J., Kwak, K., Xie, J. & Fayer, M. D. Ultrafast carbon-carbon single-bond rotational isomerization in room-temperature solution. Science 313, 1951–1955 (2006).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Koo, L. S., Immoos, C. E., Cohen, M. S., Farmer, P. J. & Ortiz de Montellano, P. R. Enhanced electron transfer and lauric acid hydroxylation by site-directed mutagenesis of CYP119. J. Am. Chem. Soc. 124, 5684–5691 (2002).
Acknowledgements
T.R.W. thanks the NCCR Catalysis (grant no. 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. Additional funding was provided by the NCCR Molecular Systems Engineering (grant no. 200021_178760). We acknowledge T. Weymuth and M. Reiher for help with the computational studies. We acknowledge J. Stropp and D. Klose for their assistance with preliminary electron paramagnetic resonance studies. We acknowledge T. Kardashliev and Z. Zou for providing original plasmids of various haemoproteins. We acknowledge J. Zurflüh for the help with the GC-MS analysis.
Author information
Authors and Affiliations
Contributions
T.R.W., X.Z. and D.C. conceived and designed the study. X.Z. and D.C. contributed equally to the synthesis of the substrates and products in Fig. 3, directed evolution of both P450BM3 and CYP119, and the mechanistic study in Fig. 5 and Supplementary Information. X.Z. and D.C. performed the catalytic experiment; X.Z. recorded the data. X.Z. and M.Á. contributed to the synthesis of substrates and products in Fig. 4 as well as the double-blind experiments. T.R.W., X.Z. and D.C. analysed the data. T.R.W., X.Z. and D.C. wrote the paper. All authors gave approval for the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Elaine O’Reilly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1
Schematic representations of active sites in natural hydrogenases (a) and selected examples illustrating the application of MHAT in total synthesis (b).
Extended Data Fig. 2 Mechanistic studies.
a, UV-Vis spectra of purified P450BM3_LQQ (black), purified P450BM3_LQQ with Na2S2O4 before (red) and after purging with CO (blue), and purified P450BM3_LQQ with PhSiH3 before (green) and after purging with CO (purple). (Inset) Expanded view of the region between 450 and 700 nm. All spectra were collected under anaerobic conditions. b, Investigation of the involvement of P450-FeII in the radical cyclization of diene 1. (Upper) Standard reaction conditions with addition of excess Na2S2O4 did not yield any pyrrolidine 2, in contrast to standard conditions without the additive. (Lower) Additional experiments were carried out in a glove box without any additive or with air or K3Fe(CN)6 as the additive. The clock icon was created with BioRender.com.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Chen, D., Álvarez, M. et al. Repurposing haemoproteins for asymmetric metal-catalysed H atom transfer. Nature 644, 381–390 (2025). https://doi.org/10.1038/s41586-025-09308-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09308-0
This article is cited by
-
Pushing the boundaries of biocatalysis
Nature Catalysis (2025)