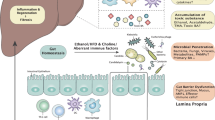
Abstract
Alcohol-use disorder and alcohol-associated liver disease (ALD) are major causes of death and liver transplantation1. The gut–liver axis has a crucial yet poorly understood role in ALD pathogenesis, which depends on microbial translocation. Intestinal goblet cells (GCs) educate the immune system by forming GC-associated antigen passages (GAPs) on activation of muscarinic acetylcholine receptor M4 (mAChR4, also known as M4), enabling sampling of luminal antigens by lamina propria antigen-presenting cells. Here we show that chronic alcohol use in humans and mice downregulates small intestinal mAChR4 and reduces GAP formation, disrupting antimicrobial immunity. This is reversed on activation of intestinal IL-6 signal transducer (IL6ST, also known as glycoprotein 130; gp130), which restores mAChR4 expression and GAP formation, enabling induction of downstream type-3 innate lymphoid cell-derived IL-22 and antimicrobial REG3 proteins. This blunts translocation of enteric bacteria to the liver, thereby conferring ALD resistance. GAP induction by GC-specific mAChR4 activation was essential and sufficient for prevention of ethanol-induced steatohepatitis. These results lay the foundation for a therapeutic approach using mAChR4 or IL6ST agonists to promote GAP formation and prevent ALD by inhibiting microbial translocation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw 16S sequencing reads are available in the NCBI SRA under BioProject accession PRJNA705611 and BioSample IDs SAMN18094194–SAMN18094231. Normalized gene expression values from duodenal bulk RNA-seq analysis are provided as log-transformed counts per million in Supplementary Table 1.
References
Lee, B. P., Vittinghoff, E., Dodge, J. L., Cullaro, G. & Terrault, N. A. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern. Med. 179, 340–348 (2019).
Llorente, C. et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat. Commun. 8, 837 (2017).
Duan, Y. et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 575, 505–511 (2019).
Bruellman, R. & Llorente, C. A perspective of intestinal immune-microbiome interactions in alcohol-associated liver disease. Int. J. Biol. Sci. 17, 307–327 (2021).
Raya Tonetti, F. et al. Gut–liver axis: recent concepts in pathophysiology in alcohol-associated liver disease. Hepatology https://doi.org/10.1097/HEP.0000000000000924 (2024).
Knoop, K. A., McDonald, K. G., McCrate, S., McDole, J. R. & Newberry, R. D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198–210 (2015).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012).
Kulkarni, D. H. et al. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 13, 271–282 (2020).
Gustafsson, J. K. et al. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. eLife 10, e67292 (2021).
Cario, E. et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164, 966–972 (2000).
Koff, J. L., Shao, M. X., Ueki, I. F. & Nadel, J. A. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L1068–L1075 (2008).
Kulkarni, D. H. et al. Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal Immunol. 11, 1103–1113 (2018).
Hartmann, P. et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology 58, 108–119 (2013).
Kuhn, K. A. et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 11, 357–368 (2018).
Llorente, C. Isolation of myenteric and submucosal plexus from mouse gastrointestinal tract and subsequent co-culture with small intestinal organoids. Cells 13, 815 (2024).
Llorente, C. The imperative for innovative enteric nervous system-intestinal organoid co-culture models: transforming GI disease modeling and treatment. Cells 13, 820 (2024).
Findeisen, M. et al. Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 574, 63–68 (2019).
McDonald, K. G., Schill, E. M., Joyce, E., Floyd, A. N. & Newberry, R. D. Maternal antibiotic treatment dysregulates goblet cell-associated antigen passages in neonatal mice. J. Immunol. 210, 72.41 (2023).
Taniguchi, K. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519, 57–62 (2015).
Todoric, J. et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab. 2, 1034–1045 (2020).
Denning, T. L. et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 187, 733–747 (2011).
Takatori, H. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41 (2009).
Wang, L. et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 19, 227–239 (2016).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl Acad. Sci. USA 99, 12651–12656 (2002).
Shi, X. Z. & Sarna, S. K. Inflammatory modulation of muscarinic receptor activation in canine ileal circular muscle cells. Gastroenterology 112, 864–874 (1997).
Bertola, A., Mathews, S., Ki, S. H., Wang, H. & Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637 (2013).
Bubser, M. et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem. Neurosci. 5, 920–942 (2014).
Pancani, T. et al. Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc. Natl Acad. Sci. USA 112, 14078–14083 (2015).
Gould, R. W. et al. Cognitive enhancement and antipsychotic-like activity following repeated dosing with the selective M4 PAM VU0467154. Neuropharmacology 128, 492–502 (2018).
Knoop, K. A., McDonald, K. G., Kulkarni, D. H. & Newberry, R. D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65, 1100–1109 (2016).
Knoop, K. A. et al. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 8, 400–411 (2017).
Gregorieff, A., Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015).
Yu, S. et al. Paneth cell multipotency induced by Notch activation following injury. Cell Stem Cell 23, 46–59 (2018).
Romera-Hernandez, M. et al. Yap1-driven intestinal repair is controlled by group 3 innate lymphoid cells. Cell Rep. 30, 37–45 (2020).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 12, 715–723 (2011).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Talbot, J. et al. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 579, 575–580 (2020).
Nystrom, E. E. L. et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 372, eabb1590 (2021).
Walker, L. C. et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: converging evidence from humans and rodents. Biol. Psychiatry 88, 898–909 (2020).
Stoll, K., Hart, R., Lindsley, C. W. & Thomsen, M. Effects of muscarinic M1 and M4 acetylcholine receptor stimulation on extinction and reinstatement of cocaine seeking in male mice, independent of extinction learning. Psychopharmacology 235, 815–827 (2018).
Schmidt, L. S. et al. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology 216, 367–378 (2011).
de la Cour, C. et al. Enhanced self-administration of alcohol in muscarinic acetylcholine M4 receptor knockout mice. Eur. J. Pharmacol. 746, 1–5 (2015).
Yang, Q., Bermingham, N. A., Finegold, M. J. & Zoghbi, H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 (2001).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Kingwell, K. Muscarinic drugs breathe new life into schizophrenia pipeline. Nat. Rev. Drug Discov. 23, 647–649 (2024).
Nguyen-Khac, E. et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment. Pharmacol. Ther. 28, 1188–1198 (2008).
Jeon, J. et al. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J. Neurosci. 30, 2396–2405 (2010).
Tomura, M. et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl Acad. Sci. USA 105, 10871–10876 (2008).
Hsu, C. L. et al. Differences in bacterial translocation and liver injury in ethanol versus diet-induced liver disease. Dig. Dis. Sci. 68, 3059–3069 (2023).
Zhou, R. et al. Intestinal α1-2-fucosylation contributes to obesity and steatohepatitis in mice. Cell. Mol. Gastroenterol. Hepatol. 12, 293–320 (2021).
Morton, A. M. et al. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc. Natl Acad. Sci. USA 111, 6696–6701 (2014).
Maccioni, L. et al. Duodenal CD8+ T resident memory cell apoptosis contributes to gut barrier dysfunction and microbial translocation in early alcohol-associated liver disease in humans. Aliment. Pharmacol. Ther. 56, 1055–1070 (2022).
Yan, A. W. et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 53, 96–105 (2011).
Fouts, D. E., Torralba, M., Nelson, K. E., Brenner, D. A. & Schnabl, B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J. Hepatol. 56, 1283–1292 (2012).
Chen, P. et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 148, 203–214 (2015).
Haas, B. J. et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504 (2011).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl Acad. Sci. USA 108, 4516–4522 (2011).
Aymanns, S., Mauerer, S., van Zandbergen, G., Wolz, C. & Spellerberg, B. High-level fluorescence labeling of Gram-positive pathogens. PLoS ONE 6, e19822 (2011).
Maeda, H. et al. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol. Med. Microbiol. 39, 81–86 (2003).
Knoop, K. A. et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2, eaao1314 (2017).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Acknowledgements
This study was supported in part by NIH grants R01 AA029106, R21 AA030654, P30 AR073761 and D34 HP31027 to the UC San Diego’s Hispanic Center of Excellence; the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases (ALPD) and Cirrhosis (P50 AA011999) funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and its Animal Core facilities; the American Association for the Study of Liver Diseases (AASLD) Pinnacle Research Awards in Liver Disease (8998GA); and the Isenberg Endowed Fellowship jointly awarded by the Pilot/Feasibility Program of the San Diego Digestive Diseases Research Center (SDDRC), the Hellman Family Foundation (P30 DK120515) (to C.L.). Additional support was provided by NIH grant K12 HD105271, UC San Diego Altman Clinical and Translational Research Institute (ACTRI)/NIH grant KL2 TR001444, AASLD Pinnacle Research Award in Liver Disease PNC22-159963 (to P.H.), NIH grant K99 AA031328 and CTORA23-208366 from the American Association for the Study of Liver Diseases Foundation (to C.L.H.), JSPS KAKENHI (JP 15K21775, JP 20H03758), AMED (PRIME) under grant number JP 18gm6210008/19gm6210008/20gm6210008/21gm6210008 (to K.T.), Fond National de Recherche Scientifique Belgium (J.0146.17 and T.0217.18) and Action de Recherche Concertée (ARC 18/23-092), Université Catholique de Louvain, Belgium (to P.S.) and ZONMW-VENI grant 2023 (09150162310148) to A.S.M.; NIH grants U01 027681, R01 CA234128, R37 AI043477 (to M.K.); R01 AA24726, R37 AA020703, U01 AA026939, award number BX004594 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and a Biocodex Microbiota Foundation Grant (to B.S.); and services provided by NIH centers P30 DK120515 and P50 AA011999. M.A.F. was supported by an L3 Investigator grant from the National Health & Medical Research Council of Australia (NHMRC grant no. APP1194141).
Author information
Authors and Affiliations
Contributions
C.L. was responsible for the acquisition, analysis and interpretation of data, study concept, design, drafting and editing of the manuscript. F.R.T. was responsible for data acquisition, analysis and interpretation. R. Bruellman, R. Brea, N.P., P.H., L.M., H.H., N.C., J.L., A.E., M.F.F., A.S.M., A.A., R.Z., Y.D. and P.Z. assisted with data acquisition. B.G. was responsible for 16S rRNA-seq data analysis. C.L.H. provided clinical advice. M.A.F. provided the IL6ST-activating cytokine IC7Fc. K.T. created the gp130Act/IEC mouse model. R.D.N. and D.A.B. provided scientific advice and technical support. D.E.F. was responsible for 16S rRNA-seq. P.S. was responsible for collection of human samples. B.S. and M.K. were responsible for study concept and design, study supervision and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.S. has consulted for Ferring Research Institute, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals, Surrozen and Takeda. P.H.’s institution, UC San Diego, has received research support from Nterica Bio. B.S.’s institution, UC San Diego, has received research support from Axial Biotherapeutics, BiomX, ChromoLogic, CymaBay Therapeutics, NGM Biopharmaceuticals, Prodigy Biotech and Synlogic Operating Company. B.S. is the founder of Nterica Bio. UC San Diego has filed several patents with C.L., C.L.H., Y.D. and B.S. listed as inventors related to this work. M.A.F. is the founder and shareholder of Celesta Therapeutics. M.K. received research support from Jansen Pharmaceuticals, Merck and Gossamer Bio and is a founder of Elgia Bio. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Marco Colonna and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Chronic ethanol alters small intestinal GCs in mice and humans.
(a–c) WT mice were fed control (n = 7–9) or ethanol-containing (n = 8) Lieber DeCarli diets for 10 weeks; 3 independent experiments. (a) EGFP-E. faecalis (5x 109 CFUs) was gavaged at 3 and 0.5 h before euthanasia (n = 6); 3 independent experiments. Representative sections of EGFP (green), TMR-dextran indicating GAPs (red), and DAPI (blue) stained SI sections. Left panel: scale bar = 100 μm. Right panel (amplification of the dashed white oval on left panel): scale bar = 12 μm. Arrowheads indicate duodenal GAPs. (b) GCs were visualized using Muc2 and Periodic Acid/Schiff (PAS) staining. Percentage of duodenal Muc2-stained area in the proximal SI (PSI) and PAS-stained cells were enumerated in each villus in the PSI and each colonic crypt. (c) Representative sections of Muc2 (red; GCs) and DAPI (blue) IF images (scale bar = 250 μm), and representative PAS-stained sections showing an increased number of GCs in the PSI and colon of ethanol-fed mice (scale bar = 100 μm). (d, e) Number of positively PAS-stained cells per villus of duodenal biopsies from controls (n = 8) and patients with AUD (n = 15), and representative PAS-stained sections. Scale bar = 100 μm. (f) GSEA of GC-related genes (GALNT10, UGT1A10, PLEKHS, GALNT14, CEACAM6, MOGAT2, FUT3, USH1C, ELF3, POF1B, KRT20, FXYD3, SMIM22, FUT2, ZG16, GALNT6, PRSS8, CEACAM5, EPCAM, CDH17, TMPRSS2, C1GALT1, C1ORF210, MUC13, PKP3, B3GNT6, TMPRSS4, MST1R, C1ORF116, MUC17, SLC44A4, AP1M2, PHGR1, B3GNT3, MISP, LAD1, ST6GALNAC4, LIPH, TSPAN8, CEACAM1, AGR3, SLC26A3, ACE2, PPP1R14D, CAPN8, TPSG1, MUC5AC, MUC2) in AUD and control subjects. (g) Volcano plot of the upregulated terms in patients with AUD from the PanglaoDB Augmented 2021 gene set. Each point represents a single term from the enrichment results. P values were determined by the two-sided unpaired Student’s t-test (b, d), or Fisher’s exact test with Benjamini–Hochberg adjustment (FDR) (g). NES represents the strength; q-value was determined as the maximum Benjamini–Hochberg-adjusted p value across Fisher’s test, fGSEA, Camera, and GSVA/limma (f). Results are expressed as mean ± s.e.m. *P < 0.05, **P (or q) < 0.01. The illustrations in a, b, d, and g were created using BioRender.
Extended Data Fig. 2 IL6ST/gp130 engagement stimulates mAChR4 expression and GAP formation.
(a–k) WT mouse SI organoids were co-cultured with submucosal and myenteric neurons15. (a) Representative immunofluorescence (IF) image showing TUBB3 (red) marking enteric neurons, MUC2 (green)-stained GCs, and DAPI (blue) marking nuclei. The dashed white rectangle shows the magnified area with a neuron-GC interaction. Scale bars: left=100 μm; right=20 μm; n = 7, 3 independent experiments. (b,c) Chrm4 and Pik3r6 mRNAs in SI organoids co-cultured with enteric neurons, following IL-6 (0.2 μg ml−1) stimulation for 24 h -/+ ethanol (10 mM) (n = 6–7 conditions per group, 3 technical replicates). (d–f) Q-PCR analysis of Chrm4, Reg3g, and Reg3b mRNAs in SI organoids from 8- to 12-week-old WT mice (n = 6, with 2 technical replicates). Organoids were stimulated with IL-6 or IL-22 (0.1 and 0.5 μg ml−1) for 24 h in the absence of ethanol. (g) Relative mAChR4 amounts in WT SI organoids incubated with or without IL-6 (0.5 μg ml−1) (n = 3, 2 technical replicates) for 24 h in the absence of ethanol and representative mAChR4 IBs. (h–k) SI organoids co-cultured with enteric neurons (h–i) or without enteric neurons (n = 2–3, 3 technical replicates) (j–k) were nano-injected with fluorescent OVA and treated with IL-6 (0.2 μg ml−1) for 1 h -/+ tropicamide (0.02 μg ml−1) during serum starvation. (h, j) GAPs per organoid. (i, k) IF of above organoids (OVA, green), GCs were stained with UEA-I (magenta), neurons with TUBB3 (white), and nuclei with DAPI (blue). Dashed white rectangles = magnified area. Blue dashed lines = organoids. Dashed white circles = OVA-filled GC. Scale bars: upper panels=100 μm; lower panels=20 μm. Diagonal ticks indicate a break in the y-axis scale (b, c, e, and f). P or q values were determined by one-way ANOVA with Tukey’s post hoc test (d, h, and j), Krustal-Wallis with BKY FDR (e–f), two-sided unpaired Student’s t-test (b, g), or Mann-Whitney U-statistic test (c). Results are expressed as mean ± s.e.m. P (or q) *< 0.05, **< 0.01, ***< 0.001. Q when applying the BKY FDR method.
Extended Data Fig. 3 IL6ST engagement stimulates mAChR4 expression and GAP formation while ethanol-mediated mAChR4 inhibition is driven via MAPK.
(a, b) WT mouse SI loops were injected with TMR-dextran and a single stimulus–either ethanol (10 mM), IL-6 (0.2 μg μl−1), VU0467154 (5 ng μl−1), or tropicamide (0.02 μg μl−1)–and incubated in DMEM containing the same stimuli for 30 min. GAPs are Muc2-positive dextran-filled columns traversing the nucleated epithelium. n = 6–8, 3 technical replicates (a) GAPs per villus. (b) Representative sections stained with TMR-dextran (red), Muc2 antibody (green), and DAPI (blue). Scale bar = 25 μm. (c) SI organoids from WT mice co-cultured with submucosal and myenteric neurons15 were treated with IC7Fc (0.5 μg ml−1) or control Ig (anti-HIV gp120) (0.5 μg ml−1) for 24 h in the absence of ethanol during serum starvation. Chrm4 mRNA was measured by qPCR (n = 8 per group, 3 technical replicates). (d) To study GAP formation, 2 cm SI loops from WT mice were injected with TMR dextran in combination with control Ig (anti-HIV gp120) (0.5 μg ml−1) or IC7Fc (0.5 μg ml−1). (n = 7–8, with 3 technical replicates). The loops were then incubated in DMEM containing the same stimuli for 30 min. Representative images of TMR-dextran (red) showing GAPs (white arrowheads), Muc2 (green), and DAPI (blue) stained SI sections. Scale bar = 25 μm. (e) Representative duodenal p-p44/42 MAPK IBs from SI organoids from WT mice after 12 h ethanol treatment (0, 10, and 50 mM) (n = 2, 2 technical replicates). (f) Quantification of β-actin normalized duodenal p-p44/42 MAPK amounts from WT mice fed control (n = 7) or ethanol-containing (n = 6) Lieber DeCarli diets for 10 weeks and representative IBs; 3 technical replicates. (g) Chrm4 mRNA in SI organoids from WT mice after 24 h ethanol treatment (10 mM) -/+ 10 µg ml−1 U-0126 (MAPK inhibitor) added for 30 min during serum starvation (n = 8 per group, 4 technical replicates). Diagonal ticks indicate a break in the y-axis scale (c, g). P values were determined by one-way ANOVA with Tukey’s post hoc test (a), or either the two-sided unpaired Student’s t-test (c–d, f), or Mann-Whitney U-statistic test (g). Results are expressed as mean ± s.e.m. *P < 0.05, ** < 0.01, *** < 0.001, **** < 0.0001. The illustrations in b and f were created using BioRender.
Extended Data Fig. 4 IL6ST activation prevents ethanol-induced liver disease and alters GC biology.
(a–d; g–n) WT and gp130Act/IEC littermates were fed control (n = 4–7) or ethanol-containing (n = 3–27) Lieber DeCarli diet for 10 weeks. A group of littermate mice was treated with tropicamide (20 mg kg−1) during the last 29 days as an interventional approach (n = 8–19); 50 independent experiments. (a) Representative ORO-stained liver sections. Scale bar = 100 μm. (b) Quantification of ORO staining. (c) Intake of liquid diet. (d) Ethanol in plasma. (e–f) Goblet cell-specific Spdef and Retnlb mRNAs are enriched in isolated SI GCs (iGC) relative to the entire PSI; 3 independent experiments. (g) (left panel) Choline acetyltransferase (CHAT) near GCs marking cholinergic innervations, and (right panel) positive (UEA-I) GCs per mm2 in the PSI. (h) Representative IF image showing CHAT expression (pink) near UEA-I (green)-labelled GCs whose membranes were stained for EpCAM (white). Nuclei were counterstained with Hoechst (blue). Yellow arrowheads indicate CHAT-expressing GCs. 40 μm sections. Scale bar=50 μm. (i) Percentage of CHAT in duodenal GAPs. (j) Representative IF image showing TMR-dextran (red) in GAPs, CHAT (pink) marking cholinergic innervations of GCs with open GAPs, Wheat Germ Agglutinin (WGA) and UEA-I staining of glycans in GCs (green and blue, respectively), epithelial cell membranes are marked by EpCAM (white). 5 μm sections. Scale bar=20 μm. (k) Expression of TUBB3 in innervated PSI GCs. (l) A representative IF image showing TUBB3-stained enteric neurons (red), GCs labelled with UEA-I (green), and epithelial cell membranes marked by EpCAM (white). Nuclei are counterstained with Hoechst (blue). 40 μm sections. Yellow arrowheads indicate cholinergic-innervated GCs. Scale bar=25 μm. (m) Number of Muc2-positive GCs per villus in the PSI. (n) Representative sections stained with Muc2 (red) showing GCs and DAPI (blue) for nuclei. 5 μm sections. Scale bar=20 μm. (o, p) Co-housed WT and gp130Act/IEC littermates were fed ethanol-containing Lieber DeCarli diet (n = 12–13) following the chronic and binge ethanol-feeding model for 10 days. (o) Plasma ALT in co-housed mice. (p) Hepatic triglycerides. Diagonal ticks indicate a break in the y-axis scale (e, f, o, p). P values were determined using two-way ANOVA with Tukey’s test (c), one-way ANOVA with Tukey’s test (b, d, g–left panel, k and m), with the BKY FDR test (g–right panel), Krustal-Wallis with Dunn’s (k) or either a two-sided unpaired Student’s t-test (i, o and p) or Mann-Whitney U test (e, f). Results are expressed as mean ± s.e.m. P (or q) *< 0.05, **< 0.01, ***< 0.001, ****<0.0001. Q when applying the BKY FDR method. The illustration in n was created using BioRender.
Extended Data Fig. 5 IL6ST signalling in IEC regulates SI LP-APCs.
(a–i) WT and gp130Act/IEC littermates were fed control (n = 10–11) or ethanol-containing (n = 8–12) Lieber DeCarli diet for 10 weeks. A group of littermates was treated with tropicamide (20 mg kg−1) during the last 29 days as an interventional approach (n = 5–11); 14 independent experiments. LP immune cells were isolated and stimulated with flagellin (100 ng ml−1) for 2.5 h. Gating strategy (Supplementary Fig. 6a) (a–f, h–i). (a) Frequencies at the gate of APCs (CD45+MHCII+CD11c+) within the total mononuclear phagocytes-enriched population. (b) Representative APC scatter plots. (c) Total APC (CD45+MHCII+CD11c+) number. (d) Total APC (CD45+MHCII+CD11c+) number according to CD103, CD11b, and CX3CR1 staining. (e–f) Total number of IL-23 and IL-10 expressing cells amongst all APC subsets. (g) Bacterial 16S relative to Gapdh RNA in the liver. (h–i) Total number of IL-23 and IL-10 expressing cells within all APC subsets. (j–o) MLN-immune cells from Kaede-WT and Kaede-gp130Act/IEC littermate mice fed control and alcohol-containing Lieber DeCarli diets for 10 weeks (n = 4–10) were photoconverted from green to red, and their migration to the liver was monitored; 6 independent experiments. Gating strategy (Supplementary Fig. 7a,b) (j–o). (j–m) Frequencies within total mononuclear phagocytes and numbers of the sum of the total Kaede green and red (total) (j, l) and Kaede red (MLN-migrated) (k, m) positive APCs (CD45+MHCII+CD11c+) categorized according to CD103, CD11b, and CX3CR1 staining present in the liver. (n, o) Number of total (CD3−RORγt+) ILC3 and Kaede-red MLN-migrated ILC3 (red) in the liver. Diagonal ticks indicate a break in the y-axis scale (c–i). P values were determined using two-way ANOVA with Tukey’s test (c, d–upper left panel) or BKY FDR test (d–upper right panel and lower left and right panels), or Kruskal Wallis test with the Dunn’s test (a– right panel, f) or with BKY FDR test (a-left panel, h–i), and either a two-sided unpaired Student’s t-test (g, l, o) or Mann-Whitney U test (e, j–k, m–n). Results are expressed as mean ± s.e.m. P (or q) *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. Q when applying the BKY FDR method. The illustration in o was created using BioRender.
Extended Data Fig. 6 IL6ST signalling in IECs increases SI LP-APCs and Tregs and blunts ethanol-induced steatohepatitis.
(a–j, l–o) WT and gp130Act/IEC littermates were fed either control (n = 3–15) or ethanol-containing (n = 3–44) Lieber DeCarli diet for 10 weeks. (f–h, l–o) A group of littermate mice was treated with tropicamide (20 mg kg−1) during the last 29 days as an interventional approach (n = 6–17); 50 independent experiments. (a, b) Number of duodenal GAPs per mm². (c, d) Number of CD11c+ APCs per mm² in the PSI. (e) Representative IF image showing TMR-dextran (red) in GAPs, CD11c (pink), WGA (green), and UEA-I (blue) staining of duodenal GCs, and epithelial cell membranes are labelled with EpCAM (white). White drawings show GAPs in communication with CD11c+ APCs. Images are 5 μm sections. Scale bar=20 μm. (f–h) Isolated SI LP-immune cells were stimulated with PMA (10 ng ml−1) plus ionomycin (500 ng ml−1) for 4 h. Gating strategy (Supplementary Fig. 8a) (f–h). (f) Frequencies at the gate of Tregs (CD4+CD25+FOXP3+) within the total lymphocyte population. (g) Representative Treg scatter plots. (h) Total Treg (CD4+CD25+FOXP3+) number. (i) β-actin-normalized duodenal IL-10 and representative blots. (j) Cd274 mRNA amounts. (k) Volcano plot of the downregulated terms in patients with AUD from the PanglaoDB Augmented 2021 gene set. Each point represents a single term from the enrichment results. Blue text indicates pathways associated with relevant immune cells and neuronal regulation. (l, m) Representation of already described data in Fig. 4, comparing all groups in one graph. (l) Duodenal GAPs per villus. (m) Plasma ALT. (n–o) Liver Cxcl5 and Ccl2 mRNA amounts. Diagonal ticks indicate a break in the y-axis scale (f, h, i, m–o). P values were determined using two-way ANOVA with Tukey’s test (f, l–m) or BKY FDR test (h), one-way ANOVA with the BKY FDR test (j, n–o), and either the two-sided unpaired Student’s t-test (b, d), unpaired Welch’s t-test (a) or Mann-Whitney U test (c, i), or Fisher’s exact test with Benjamini–Hochberg adjustment (FDR) (k). Results are expressed as mean ± s.e.m. P (or q) *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. Q when applying the BKY FDR method. The illustration in o was created using BioRender.
Extended Data Fig. 7 Top genes that correlate with CHRM4 expression.
(a–b) RNA-seq analysis was performed as in Fig. 1 (AUD, n = 44; controls, n = 16). (a) Heatmaps displaying Pearson correlation (r) values for the top 30 genes that positively and negatively correlate with CHRM4 mRNA. (b) Linear regression and correlation coefficients (R) depicting co-expressed gene pairs across the samples. The straight, bold line represents the linear regression fit, and the lines above and below the bold line represent the 99% prediction bands. (c–d) Chrm4 integrated density in GCs determined by RNAscope from 8- to 12-week-old Chrm4ΔGC and WT littermates (n = 3; 2 independent experiments). (d) Representative image showing Chrm4 mRNA (red), EpCAM-stained epithelial cell membranes (white), and DAPI-stained nuclei (blue). Yellow arrowheads: Chrm4 expressing GCs. Scale bars=20 μm. (e–j) gp130Act/IEC and Chrm4ΔGCgp130Act/IEC littermates were fed ethanol-containing Lieber DeCarli diet (n = 10–14) following the chronic and binge ethanol-feeding protocol for 10 days, 11 independent experiments. (e) Hepatic Acta2 mRNA. (f) Plasma ethanol. (g–h) Hepatic Adh1 and Cyp2e1 mRNAs. (i–j) Duodenal Muc2 and Spdef mRNAs. Diagonal ticks indicate a break in the y-axis scale (e). P values from two-tailed t-tests on regression coefficients from multiple (a) or simple linear regression (b) and by either a two-sided unpaired Student’s t-test (f, h–i) or unpaired Welch’s test (c–e) or Mann-Whitney U test (g, j). Results are mean ± s.e.m. *P < 0.05, ****P < 0.0001. The illustrations in b and j were created using BioRender.
Extended Data Fig. 8 IEC-specific IL6ST ablation exacerbates ethanol-induced liver disease, inhibits SI GAP formation, and alters the LP-immune landscape.
(a–u) WT and gp130ΔIEC littermates were fed either control (n = 4–13) or ethanol-containing (n = 4–49) Lieber DeCarli diets for 10 weeks. A group of gp130ΔIEC mice were treated with the mAChR4 PAM VU0467154 (5 mg kg−1) dissolved in the diet during the last 29 days (n = 4–19); 65 independent experiments. (a) Plasma ALT. Note that WT samples were also used as controls in Fig. 5c, all littermates. (b) Representative H&E-stained liver sections. Scale bar=200 μm. (c) Quantification of ORO staining. WT samples were also used as controls in Fig. 5e. (d) Representative ORO-stained liver sections. Scale bar = 100 μm. (e–g) Liver Ccl2, Cxcl5, and Col1a1 mRNA amounts. (h) Survival rate curves. Statistical analysis was performed using the Gehan-Breslow-Wilcoxon test (Chi-square=4.33, P = 0.0374). (i) Plasma ethanol. WT samples were also used in Extended Data Fig. 9p. (j) Liquid diet intake. WT samples were also used as controls in Extended Data Fig. 9o. (k) Duodenal GAPs per villus. WT samples were also used as controls in Fig. 5a. (l) Representative images of TMR-dextran (red) showing GAPs (white arrowheads), Muc2 (green), and (DAPI) (blue) stained SI sections. Scale bar=25 μm. (m–n) Chrm4 mRNA amounts in isolated GCs (m) and duodenal samples (n). (o) Percentage of Muc2-stained area in the PSI. (p) Representative sections stained with Muc2 (red) showing GCs, and DAPI (blue) stained nuclei. 5 μm sections. Scale bar=20 μm. (q) Number of CFUs of anaerobically cultured bacteria from sterile collected MLN (100 mg) and liver (200 mg). WT samples were also used as WT controls in Fig. 5m (middle and right panels). (r–u) Isolated LP cells were stimulated with PMA (10 ng ml−1) plus ionomycin (500 ng ml−1) for 4 h. (r) Frequencies of CD4+/CD25+/FOXP3+ Tregs within total lymphocytes and total numbers. Gating strategy (Supplementary Fig. 8a). (s) Duodenal Il10 mRNA. (t) Frequencies of CD8+ Tregs (CD45+/CD8+/CD25+/FOXP3+) within total lymphocytes and total numbers. Gating strategy (Supplementary Fig. 8b). (u) Total IL-10 expressing CD8+ Treg (CD45+/CD8+/CD25+/FOXP3+/IL-10+) numbers. Diagonal ticks indicate a break in the y-axis scale (a, e–g, q–s). P values were determined using two-way ANOVA with Tukey’s test (j, q–left). or the BKY FDR (c, q–right), one-way ANOVA with Tukey’s test (i) or the BKY FDR (a, k, o), Kruskal-Wallis with the BKY FDR test (r), or either the two-sided unpaired Student’s t-test (s, t–left panel, u), unpaired Welch’s test (n), or the Mann-Whitney U test (e–g, m, t–right panel). Survival curves were compared using the Gehan–Breslow–Wilcoxon test (h). Results are presented as mean ± s.e.m. P (or q) *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. Q when applying the BKY FDR method. The illustration in p was created using BioRender.
Extended Data Fig. 9 IEC-specific IL6ST ablation impairs protective SI immune responses, while mAChR4 agonism reverts it.
(a–m) WT and gp130ΔIEC littermates were fed ethanol-containing (n = 4–22) Lieber DeCarli diet for 10 weeks; 8 independent experiments. Isolated LP cells were stimulated with PMA (10 ng ml−1) plus ionomycin (500 ng ml−1) for 4 h (a–c, m–n) or with flagellin (100 ng ml−1) for 2.5 h (k–l). Gating strategy (Supplementary Fig. 9a). (a) Frequencies at the gate of ILC3s (CD45+/CD4–/RORγt+) within total lymphocytes and total numbers. (b) Representative frequencies at the gate and within total lymphocytes and numbers of IL-22 expressing ILC3s. (c) Duodenal Il22 mRNA. (d) β-actin-normalized duodenal REG3B/G proteins and representative IBs. (e–m) gp130ΔIEC littermates were fed an ethanol-containing Lieber DeCarli diet (n = 8–17) for 10 weeks -/+ the mAChR4 PAM VU0467154 (5 mg kg−1), dissolved in the diet during the last 29 days; 7 independent experiments. (e) Liver triglycerides. (f) Representative H&E-stained liver sections. Scale bar=200 μm. (g–h) Liver Cxcl1 and Col1a1 mRNAs. (i) Representative TMR-dextran (red) showing GAPs (white arrowheads), Muc2 (green), and DAPI (blue) stained SI sections. Scale bar=25 μm. (j–k) Frequencies of APC subsets within the total mononuclear phagocytes-enriched population were gated based on CD45, MHCII, CD11c, CD103, CD11b, and CX3CR1 staining. Gating strategy (Supplementary Fig. 6a). (l) Frequencies at the gate of CD8+ Tregs (CD45+/CD8+/CD25+/FOXP3+) within the total lymphocyte population and total numbers. (m) Total number of IL-10 expressing CD8+ Tregs. Gating strategy (Supplementary Fig. 8b) (l–m). Note that the gp130ΔIEC samples were also used in Extended Data Fig. 8t–8u, all littermates. (n–q) WT mice were fed control (n = 7–11) or ethanol-containing (n = 4–27) Lieber DeCarli diets for 10 weeks -/+ VU0467154 (5 mg kg−1), dissolved in the diet during the last 29 days, 7 independent experiments. (n) Hepatic Col1a1 mRNA. (o) Liquid diet intake. (p) Ethanol in plasma. (q) The number of APC subsets within total mononuclear phagocytes based on CD45, MHCII, CD11c, CD103, and CD11b staining. Gating strategy (Supplementary Fig. 6a). (r–u) mAChR4Act/GC littermates were fed ethanol-containing Lieber DeCarli diet (n = 6–27) following the chronic and binge ethanol-feeding model for 10 days and treated with vehicle or 200 μg kg−1 DCZ in their diet; 14 independent experiments. (r) Plasma ethanol. (s–t) Liver Adh1 and Cyp2e1 mRNA amounts. (u) Duodenal Chrm4, Pik3ca, Pik3r1, Pik3r6, Akt1, Rack1, Ryr3, Muc2, Retnlb, Spdef, Fut2, Foxp3, Il10, Il22, Reg3b, Reg3g mRNA amounts. Diagonal ticks indicate a break in the y-axis scale (a, b, e, g–h, j–m, q). P values were determined using one-way ANOVA with Tukey’s test (o), or either the two-sided unpaired Student’s t-test (a–left panel, c, d–left panel, g, j, l–left, p, r–s), unpaired Welch’s test (a–right panel, e, h, n, q, u–Pikr1, Pikr6, Akt1, Rack1, Retnlb, Spdef, Fut2, Foxp3, Reg3g) or the Mann-Whitney U test (b, d–right panel, k, l–right, m, t, u–Chrm4, Pik3ca, Ryr3, Muc2, Il10, Il22, Reg3b). Results are presented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. The illustrations in m, q, and t were created using BioRender.
Extended Data Fig. 10 Goblet cell-specific mAChR4 ablation enhanced MASLD development in mice.
(a–i) WT (n = 12) and Chrm4ΔGC (n = 14) littermate mice were fed Western diet, together with glucose (18.9 g L−1) and fructose (23.1 g L−1) in the drinking water (Western diet; WD) for 24 weeks; 11 independent experiments. (k–s) Same protocol was followed for mAChR4Act/GC mice treated with vehicle (n = 12) or 400 μg kg−1 DCZ (n = 6); 6 independent experiments. (a, j) Body weight increase (left panels), area under the curve (AUC) of the body weight increase (right panels). (b, k) Cumulative food intake. (c, l) Cumulative liquid intake. (d) Liver weight at 24 weeks. (e, m) Plasma ALT. (f) Hepatic triglycerides. (g, n) Representative images of H&E-stained liver tissue. Scale bar=100 μm. (h, q) Quantification of Sirius red-stained liver area. (i, r) Representative images of Sirius red-stained liver tissue. Scale bar=100 μm. (o, p) Hepatic Cd14 and Timp1 mRNA levels. Diagonal ticks indicate a break in the y-axis scale (e). P values were determined using two-way ANOVA with BKY FDR test (a–left panel, b–c, j–left panel, k–l), or either the two-sided unpaired Student t-test (d, f, h, j-right panel, q), unpaired Welch’s test (m, o–p) or Mann-Whitney U (a–right panel, e) test. P (or q) *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. Q when applying the BKY FDR method.
Supplementary information
Supplementary Figures
Supplementary Figs. 1–10.
Supplementary Table 1
Data and statistics for Figs. 1–5.
Supplementary Table 2
Data and statistics for Extended Data Figs. 1–10 and Supplementary Fig. 1.
Supplementary Table 3
Sequences of qPCR primers.
Rights and permissions
About this article
Cite this article
Llorente, C., Raya Tonetti, F., Bruellman, R. et al. mAChR4 suppresses liver disease via GAP-induced antimicrobial immunity. Nature 646, 180–189 (2025). https://doi.org/10.1038/s41586-025-09395-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09395-z