Abstract
Migration of transplanted allogeneic myeloid cells into the brain following systemic haematopoietic stem and progenitor cell transplantation (HCT) holds great promise as a therapeutic modality to correct genetic deficiencies in the brain such as lysosomal storage diseases1,2,3. However, the toxic myeloablation required for allogeneic HCT can cause serious, life-threatening side effects, limiting its applicability. Moreover, transplanted allogeneic myeloid cells are highly vulnerable to rejection even in an immune-privileged organ like the brain. Here we report a brain-restricted, high-efficiency microglia replacement approach without myeloablative preconditioning. Contrary to previous assumptions, we found that haematopoietic stem cells are not required to repopulate the myeloid compartment of the brain environment, and Sca1− committed progenitor cells were highly efficient in replacing microglia following intracerebral injection. This finding enabled the development of brain-restricted preconditioning and avoided long-term peripheral engraftment, thus eliminating complications such as graft-versus-host disease. Evaluating its therapeutic potential, we found that our allogeneic microglia replacement method rescued the mouse model of Sandhoff disease, a lysosomal storage disease caused by hexosaminidase B deficiency. In support of the translational relevance of this approach, we discovered that human embryonic stem cell-derived myeloid progenitor cells display a similar engraftment potential following brain-restricted conditioning. Our results overcome current limitations of conventional HCT and may pave the way for the development of allogeneic microglial cell therapies for the brain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The supporting data for all graphs are provided along with this publication. If additional information for replication of this study is required, it will be available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Computational and statistical analyses were performed using freely available software packages. If additional information for replication of this study is required, it will be available from the corresponding author on reasonable request.
References
Biffi, A. et al. Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J. Clin. Invest. 116, 3070–3082 (2006).
Capotondo, A. et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc. Natl Acad. Sci. USA 109, 15018–15023 (2012).
Shibuya, Y. et al. Treatment of a genetic brain disease by CNS-wide microglia replacement. Sci. Transl. Med. 14, eabl9945 (2022).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Sankowski, R. et al. Multiomic spatial landscape of innate immune cells at human central nervous system borders. Nat. Med. 30, 186–198 (2024).
Erblich, B., Zhu, L., Etgen, A. M., Dobrenis, K. & Pollard, J. W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE 6, e26317 (2011).
Elmore, M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Huang, Y. et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 (2018).
Bennett, F. C. et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98, 1170–1183 (2018).
Cronk, J. C. et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 215, 1627–1647 (2018).
Chadarevian, J. P. et al. Engineering an inhibitor-resistant human CSF1R variant for microglia replacement. J. Exp. Med. 220, e20220857 (2023).
Ajami, B., Bennett, J. L., Krieger, C., Tetzlaff, W. & Rossi, F. M. V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 (2007).
Priller, J. et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 7, 1356–1361 (2001).
Kierdorf, K., Katzmarski, N., Haas, C. A. & Prinz, M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS ONE 8, e58544 (2013).
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007).
Wilkinson, F. L. et al. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol. Ther. 21, 868–876 (2013).
Capotondo, A. et al. Intracerebroventricular delivery of hematopoietic progenitors results in rapid and robust engraftment of microglia-like cells. Sci. Adv. 3, e1701211 (2017).
Hohsfield, L. A. et al. Effects of long-term and brain-wide colonization of peripheral bone marrow-derived myeloid cells in the CNS. J. Neuroinflammation 17, 279 (2020).
Xu, Z. et al. Efficient strategies for microglia replacement in the central nervous system. Cell Rep. 32, 108041 (2020).
Sailor, K. A. et al. Hematopoietic stem cell transplantation chemotherapy causes microglia senescence and peripheral macrophage engraftment in the brain. Nat. Med. 28, 517–527 (2022).
Loeb, A. M., Pattwell, S. S., Meshinchi, S., Bedalov, A. & Loeb, K. R. Donor bone marrow-derived macrophage engraftment into the central nervous system of patients following allogeneic transplantion. Blood Adv. 7, 5851–5859 (2023).
Mader, M. M.-D. et al. Myeloid cell replacement is neuroprotective in chronic experimental autoimmune encephalomyelitis. Nat. Neurosci. 27, 901–912 (2024).
Yoo, Y., Neumayer, G., Shibuya, Y., Mader, M. M.-D. & Wernig, M. A cell therapy approach to restore microglial Trem2 function in a mouse model of Alzheimer’s disease. Cell Stem Cell 30, 1043–1053 (2023).
Eichler, F. et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 377, 1630–1638 (2017).
Yoon, I. C., Bascou, N. A., Poe, M. D., Szabolcs, P. & Escolar, M. L. Long-term neurodevelopmental outcomes of hematopoietic stem cell transplantation for late-infantile Krabbe disease. Blood 137, 1719–1730 (2021).
Gentner, B. et al. Hematopoietic stem- and progenitor-cell gene therapy for Hurler syndrome. N. Engl. J. Med. 385, 1929–1940 (2021).
Fumagalli, F. et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet 399, 372–383 (2022).
Boucher, A. A. et al. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet J. Rare Dis. 10, 94 (2015).
Aldenhoven, M. et al. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood 125, 2164–2172 (2015).
Simard, A. R., Soulet, D., Gowing, G., Julien, J.-P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502 (2006).
Mishra, P. et al. Rescue of Alzheimer’s disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells. Cell Rep. 42, 112956 (2023).
Bryder, D., Rossi, D. J. & Weissman, I. L. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 169, 338–346 (2006).
Getts, D. R. et al. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 205, 2319–2337 (2008).
Li, S. et al. Induction of immunological tolerance to myelinogenic glial-restricted progenitor allografts. Brain 142, 3456–3472 (2019).
Lightbourn, C. O. et al. Use of post-transplant cyclophosphamide treatment to build a tolerance platform to prevent liquid and solid organ allograft rejection. Front. Immunol. 12, 636789 (2021).
Sango, K. et al. Mouse models of Tay–Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat. Genet. 11, 170–176 (1995).
Norflus, F. et al. Bone marrow transplantation prolongs life span and ameliorates neurologic manifestations in Sandhoff disease mice. J. Clin. Invest. 101, 1881–1888 (1998).
Spangenberg, E. et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 10, 3758 (2019).
Najafi, A. R. et al. A limited capacity for microglial repopulation in the adult brain. Glia 66, 2385–2396 (2018).
Hohsfield, L. A. et al. Subventricular zone/white matter microglia reconstitute the empty adult microglial niche in a dynamic wave. eLife 10, e66738 (2021).
Claeys, W. et al. Limitations of PLX3397 as a microglial investigational tool: peripheral and off-target effects dictate the response to inflammation. Front. Immunol. 14, 1283711 (2023).
Okojie, A. K. et al. Distinguishing the effects of systemic CSF1R inhibition by PLX3397 on microglia and peripheral immune cells. J. Neuroinflammation 20, 242 (2023).
Lei, F. et al. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc. Natl Acad. Sci. USA 117, 23336–23338 (2020).
Tap, W. D. et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet 394, 478–487 (2019).
Morganti, J. M., Jopson, T. D., Liu, S., Gupta, N. & Rosi, S. Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS ONE 9, e93650 (2014).
Voshart, D. C. et al. Radiotherapy induces persistent innate immune reprogramming of microglia into a primed state. Cell Rep. 43, 113764 (2024).
Mendez, I. et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat. Med. 14, 507–509 (2008).
Beegle, J., Hendrix, K., Maciel, H., Nolta, J. A. & Anderson, J. S. Improvement of motor and behavioral activity in Sandhoff mice transplanted with human CD34+ cells transduced with a HexA/HexB expressing lentiviral vector. J. Gene Med. 22, e3205 (2020).
Lee, J.-P. et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat. Med. 13, 439–447 (2007).
Chen, D. et al. Brain-wide microglia replacement using a nonconditioning strategy ameliorates pathology in mouse models of neurological disorders. Sci. Transl. Med. 17, eads6111 (2025).
Wu, J. et al. Microglia replacement halts the progression of microgliopathy in mice and humans. Science 389, eadr1015 (2025).
Doudna, J. A. The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020).
Sabatino, D. E. et al. Evaluating the state of the science for adeno-associated virus integration: an integrated perspective. Mol. Ther. 30, 2646–2663 (2022).
Goyal, S. et al. Acute myeloid leukemia case after gene therapy for sickle cell disease. N. Engl. J. Med. 386, 138–147 (2022).
Mancuso, R. et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat. Neurosci. 22, 2111–2116 (2019).
Hasselmann, J. et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron 103, 1016–1033 (2019).
Svoboda, D. S. et al. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc. Natl Acad. Sci. USA 116, 25293–25303 (2019).
Xu, R. et al. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 11, 1577 (2020).
Schaefer, B. C., Schaefer, M. L., Kappler, J. W., Marrack, P. & Kedl, R. M. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell. Immunol. 214, 110–122 (2001).
Dai, X.-M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 (2002).
Hamanaka, S. et al. Generation of transgenic mouse line expressing Kusabira Orange throughout body, including erythrocytes, by random segregation of provirus method. Biochem. Biophys. Res. Commun. 435, 586–591 (2013).
Rathinam, C. et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood 118, 3119–3128 (2011).
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019).
Wilkinson, A. C., Ishida, R., Nakauchi, H. & Yamazaki, S. Long-term ex vivo expansion of mouse hematopoietic stem cells. Nat. Protoc. 15, 628–648 (2020).
Fattorelli, N. et al. Stem-cell-derived human microglia transplanted into mouse brain to study human disease. Nat. Protoc. 16, 1013–1033 (2021).
Szot, G. L. et al. Tolerance induction and reversal of diabetes in mice transplanted with human embryonic stem cell-derived pancreatic endoderm. Cell Stem Cell 16, 148–157 (2015).
Mayumi, H. & Good, R. A. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J. Exp. Med. 169, 213–238 (1989).
Matsuura, A. et al. Cyclophosphamide-induced tolerance in fully allogeneic heart transplantation in mice. Cell. Immunol. 155, 501–507 (1994).
Dodd-o, J. M. et al. Induction of major histocompatibility complex-mismatched mouse lung allograft acceptance with combined donor bone marrow: lung transplant using a 12-hour nonmyeloablative conditioning regimen. Transplantation 100, e140 (2016).
Red blood cell lysis buffer. Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.rec390 (2006).
Guyenet, S. J. et al. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J. Vis. Exp. https://doi.org/10.3791/1787 (2010).
Ullman-Culleré, M. H. & Foltz, C. J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 49, 319–323 (1999).
Naserian, S. et al. Simple, reproducible, and efficient clinical grading system for murine models of acute graft-versus-host disease. Front. Immunol. 9, 10 (2018).
Verlaat, L. et al. Novel pre-clinical mouse models for chronic graft-versus-host disease. Front. Immunol. 13, 1079921 (2023).
Tribromoethanol (avertin). Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.rec701 (2006).
Skordos, I., Demeyer, A. & Beyaert, R. Analysis of T cells in mouse lymphoid tissue and blood with flow cytometry. STAR Protoc. 2, 100351 (2021).
Challen, G. A., Boles, N., Lin, K.-Y. K. & Goodell, M. A. Mouse hematopoietic stem cell identification and analysis. Cytometry A 75A, 14–24 (2009).
Bourgoin, C. et al. Widespread distribution of β-hexosaminidase activity in the brain of a Sandhoff mouse model after coinjection of adenoviral vector and mannitol. Gene Ther. 10, 1841–1849 (2003).
Ruifrok, A. C. & Johnston, D. A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 23, 291–299 (2001).
Landini, G., Martinelli, G. & Piccinini, F. Colour deconvolution: stain unmixing in histological imaging. Bioinformatics 37, 1485–1487 (2021).
Acknowledgements
We would like to thank all members of the Wernig laboratory, as well as A. Wilkinson, H. Nakauchi, M. Miyauchi and K. Niizuma, for helpful discussions throughout the project. We thank A. Lang and M. Vangipuram for administrative support, and we thank the FACS core at the Institute for Stem Cell Biology and Regenerative Medicine, especially C. Crumpton and C. Pan, and the Stanford Neuroscience Microscopy Service (supported by NIH NS069375) and G. Wang for technical support. We thank T. Südhof and Z. Liu for training and access to equipment for behavioural studies. We thank E. E. Graves for advice regarding small animal irradiation and calibration of the irradiation restrainer. Csf1r−/− (FVB.129×1-Csf1rtm1Ers) mice were a gift from R. Stanley (Albert Einstein College of Medicine). B6.KUO mice were generated at the University of Tokyo and were a gift from H. Nakauchi. This work was supported by the Helen C. and Robert J. Kleberg Foundation and a DISC-0 award from the California Institute for Regenerative Medicine (DISC0-13875). M.M.-D.M. was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft (DFG), MA 8492/1-1). Y.Y. was supported by the New York Stem Cell Foundation Druckenmiller Fellowship (NYSCF-D-F74). T.U. was supported by the Wu Tsai Neurosciences Institute Knight Initiative for Brain Resilience Scholar Award.
Author information
Authors and Affiliations
Contributions
Study concept and design: M.M.-D.M. and M.W. Animal experiments: M.M.-D.M., A.S. and A.T.C. Histological analyses: M.M.-D.M., A.S. and A.T.C. Cell culture: M.M.-D.M., Y.Y., A.S. and T.U. Flow cytometry experiments: M.M.-D.M. and A.S. Drafting and major editing of original manuscript: M.M.-D.M. and M.W. All authors reviewed, revised and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
M.W. is a co-founder of Neucyte, a scientific advisor for bit.bio, and co-founder and scientific advisor of Lytherian Therapeutics and Theseus Therapies. The other authors declare no competing interests. PLX5622 was provided by Plexxikon under a material transfer agreement between Stanford University and Plexxikon.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional evaluation of the effects of CSF1R inhibitors and irradiation dose.
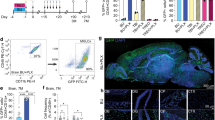
a, Experimental design. The last injection of busulfan was applied one day before transplantation, and PLX5622 diet was withdrawn on the day of transplantation. 200 ×103 B6.UBC-GFP graft cells were transplanted per recipient animal. iv, intravenous. b, Representative flow cytometry plots and gating strategy. c, Quantification of donor derived GFP+ chimerism in the peripheral blood and myeloid compartment of the contralateral brain hemisphere based on flow cytometry. n = 4, 4, 3, and 4 animals per group, respectively (from left to right). Two-sided Welch t-test. d, Evaluation of microglia depletion. Representative flow cytometry plots and gating strategy are shown for two female animals. e, Flow cytometric quantification. The brain was analyzed one day after the last PLX5622 injection. The drug was administered daily over 4 days. n = 3 animals per group. Two-sided Welch t-test. f, Flow cytometric quantification. The brain was analyzed one day after the last PLX3397 injection. The drug was administered daily over 3 days. n = 3 animals per group. Two-sided Welch t-test. g, Experimental design. 200 ×103 B6.UBC-GFP HSPC were transplanted per recipient animal. Numbers (n) represent assigned and survived animals per group. ip, intraperitoneal. h, Representative flow cytometry plots and gating strategy per experimental group. i, Flow cytometric quantification (contralateral brain hemisphere). n = 5, 4, and 1 animals per group, respectively (from left to right). Two-sided Welch t-test. j, Experimental outline. 200 ×103 B6.UBC-GFP HSPC were transplanted per recipient animal. n = 3 animals per group. k, Flow cytometric quantification (contralateral brain hemisphere). l, Immunofluorescent staining for GFP of sagittal brain sections of the ipsilateral hemisphere of two animals with irradiation+PLX3397 treatment. Shown are cortical areas of reduced donor cell engraftment density. Purple square illustrates magnified area of panel m. Scale bar = 200 µm. m, Immunofluorescent stain shows repopulated endogenous GFP− microglia and grafted GFP+ cells. Demonstrated is the border between high-density donor cell repopulated brain and an area with low chimerism. Scale bar = 50 µm. n, Experimental outline. 200 ×103 B6.UBC-GFP HSPC were transplanted per recipient animal. n = 3 animals per group. o, Flow cytometric quantification (contralateral brain hemisphere). Two-sided Welch t-test. p, Flow cytometric quantification of myeloid donor chimerism in the brain and blood 33 weeks after ICV transplantation and preconditioning with PLX3397 and 10 Gy head irradiation. n = 4 animals. Box-plot elements in Extended Data Fig. 1 represent median (center line), first and third quartiles (lower and upper hinges) and smallest/highest value with at most 1.5*IQR (inter-quartile range) from the hinge (whiskers).
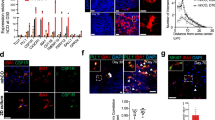
Extended Data Fig. 2 Expression of microglial homeostatic marker proteins on transplanted myeloid cells.
Representative immunofluorescent stain shows the expression of IBA1, P2RY12, and TMEM119 in endogenous GFP− microglia and grafted GFP+ cells in the cortex. Grafted cells were evaluated 25 days after transplantation. Samples of three different animals per group were examined with comparable results. Scale bar = 30 µm.
Extended Data Fig. 3 Flow cytometric gating strategies for hematopoietic stem and progenitor cells.
Flow cytometry plots and gating strategies are provided for the sorting of primary bone marrow cells (a) and ex vivo expanded HSPCs (c), as well as for the quantification of GFP+ or KUO+ fractions in myeloid cells in vitro (b, d, e). FMO, fluorescence minus one.
Extended Data Fig. 4 Flow cytometric gating strategy for peripheral blood evaluation.
Flow cytometry plots and gating strategy for the evaluation of peripheral blood cells in the context of graft rejection. BC, B cells; NK, natural killer cells; TC, T cells; TCM, central memory T cells; TEM, effector memory T cells; Tnaive, naïve T cells; Treg, regulatory T cells.
Extended Data Fig. 5 Additional characterization of allogeneic microglia replacement as a cell therapy.
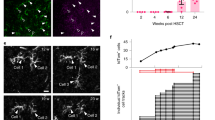
a, Engraftment of GFP+ donor cells in the cerebrum ~12 weeks after transplantation. Shown as total density and relative engrafted brain area. n = 12 CT animals. Regression line based on linear model (shaded area = confidence interval); r = Pearson correlation coefficient. Two-sided test. b, Representative GFP immunofluorescence image of a sagittal brain section. Dotted line indicates replicate in panel a. Scale bar = 500 µm. c, Body weight and conditioning score as well as GvHD score demonstrated for live animals of the experimental groups. A late increase in GvHD score especially in the KO and Mock groups coincided with neurological decline and was primarily based on weight loss and hunching. No occurrences of skin fibrosis or diarrhea were detected in this cohort. Lines represent the mean with 95% confidence interval. n = 61 animals total (WT = 19, KO = 12, Mock = 10, CT = 20). d, Representative images of in situ β-hexosaminidase determination in the cortex. A total of 27 animals were evaluated. Scale bar = 200 µm. e, Quantification of the β-hexosaminidase positive area in the whole brain. A total of 27 animals were evaluated. Two-sided Welch t-test. f, Quantification of relative time spent in the inner zone of an open field setup over 5 min. Deceased animals were excluded from the analysis. n = 19, 12, 10, and 20 animals (13 weeks) and 19, 10, 7, and 18 animals (17 weeks) in the WT, KO, Mock, and CT group, respectively. Two-sided Mann–Whitney U test. g, Correlation between brain area of engrafted cells and clinical variables. In two animals with mortality before week 18, engraftment was evaluated in necropsy tissue, and no GFP+ cells were detected (see panel h). n = 14 CT animals. Regression line based on linear model (shaded area = confidence interval); r = Pearson correlation coefficient. Two-sided test. h, Immunofluorescent images of necropsy brain tissue. Positive sample represents a CT animal with unclear mortality on day 114. Negative samples 1 and 2 represent CT animals with mortality due to neurological decline before 18 weeks of age, and no detected GFP+ myeloid cells in the brain. Red arrows illustrate present or absent colocalization of Iba1 and GFP. Scale bar = 50 µm. i, Neuro Score displayed for WT (n = 19 animals up to week 18, then 11 animals), KO (n = 12), and long-term CT (n = 5) animals. Deceased animals received a maximum score of 9. Lines represent the mean with 95% confidence interval. j, Quantification of locomotor activity in an open field setup over 5 min (performed at 13, 17, 22, 26, and 30 weeks of age). Displayed for WT (n = 19 animals up to week 17, then 11 animals), KO (n = 10 and 12 animals at weeks 17 and 13, respectively) and long-term CT (n = 5 animals up to week 17, then 4 animals) animals. Deceased animals were excluded from the analysis. Lines represent the mean with 95% confidence interval. k, Flow cytometric quantification of myeloid donor chimerism in the peripheral blood after bone marrow transplantation (BMT). syngeneic control n = 4 animals (6 weeks after BMT). allogeneic with co-stimulation blockade n = 12 animals (7×6 weeks and 5×12 weeks after BMT). l, Body weight and GvHD score. Lines represent the mean with 95% confidence interval. n = 12 animals per group. Box-plot elements in Extended Data Fig. 5 represent median (center line), first and third quartiles (lower and upper hinges) and smallest/highest value with at most 1.5*IQR (inter-quartile range) from the hinge (whiskers).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mader, M.MD., Scavetti, A., Yoo, Y. et al. Therapeutic genetic restoration through allogeneic brain microglia replacement. Nature 646, 903–912 (2025). https://doi.org/10.1038/s41586-025-09461-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09461-6