Abstract
As a negative charge carrier, the hydride ion (H−) is more energetic, polarizable and reactive than cations1. An H−-mediated electrochemical process is fundamentally different from existing systems and enables the development of innovative electrochemical devices, such as rechargeable batteries, fuel cells, electrolysis cells and gas separation membranes2. Here we developed a core-shell hydride 3CeH3@BaH2, which exhibits fast H− conduction at ambient temperature and becomes a superionic conductor above 60 °C. This hydride allows us to construct an all-solid-state rechargeable H− battery CeH2|3CeH3@BaH2|NaAlH4, which operates at ambient conditions using NaAlH4 and CeH2 as cathode and anode materials, respectively. This battery has an initial specific capacity of 984 mAh g−1 and retains 402 mAh g−1 after 20 cycles. Using hydrogen as charge carriers can avoid the formation of detrimental metal dendrites, in principle, which creates new research avenues for clean energy storage and conversion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Source data are provided with this paper.
Change history
17 October 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-09701-9
References
Yamaguchi, S. Large, soft, and polarizable hydride ions sneak around in an oxyhydride. Science 351, 1262–1263 (2016).
Zhang, W., Cao, H. & Chen, P. Hydride ion conductor: a key material for innovative energy storage and conversion. Innov. Mater. 1, 100006 (2023).
Verbraeken, M. C., Cheung, C., Suard, E. & Irvine, J. T. S. High H− ionic conductivity in barium hydride. Nat. Mater. 14, 95–100 (2015).
Zhang, W. et al. Deforming lanthanum trihydride for superionic conduction. Nature 616, 73–76 (2023).
Irvine, G. J., Smith, R. I., Jones, M. O. & Irvine, J. T. S. Order–disorder and ionic conductivity in calcium nitride-hydride. Nat. Commun. 14, 4389 (2023).
Kobayashi, G. et al. Pure H− conduction in oxyhydrides. Science 351, 1314–1317 (2016).
Fukui, K., Iimura, S., Iskandarov, A., Tada, T. & Hosono, H. Room-temperature fast H− conduction in oxygen-substituted lanthanum hydride. J. Am. Chem. Soc. 144, 1523–1527 (2022).
Takeiri, F. et al. Hydride-ion-conducting K2NiF4-type Ba–Li oxyhydride solid electrolyte. Nat. Mater. 21, 325–330 (2022).
Izumi, Y. et al. Electropositive metal doping into lanthanum hydride for H− conducting solid electrolyte use at room temperature. Adv. Energy Mater. 8, 2301993 (2023).
Ubukata, H. et al. Anion ordering enables fast H− conduction at low temperatures. Sci. Adv. 7, eabf7883 (2021).
Huiberts, J. N. et al. Yttrium and lanthanum hydride films with switchable optical properties. Nature 380, 231–234 (1996).
Vajda, P. in Handbook on the Physics and Chemistry of Rare Earths Vol. 20, chap. 137, pp. 207–292 (eds Gschneidner Jr, K. A. & Eyring, L.) (Elsevier, 1995).
Fukui, K. et al. Characteristic fast H− ion conduction in oxygen-substituted lanthanum hydride. Nat. Commun. 10, 2578 (2019).
Wang, H. L. et al. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 43, 5234–5244 (2014).
Capasso, F. Band-gap engineering: from physics and materials to new semiconductor devices. Science 235, 172–176 (1987).
Verbraeken, M. C., Suard, E. & Irvine, J. T. S. Structural and electrical properties of calcium and strontium hydrides. J. Mater. Chem. 19, 2766–2770 (2009).
Rowberg, A. J. E., Weston, L. & Van de Walle, C. G. Ion-transport engineering of alkaline-earth hydrides for hydride electrolyte applications. Chem. Mater. 30, 5878–5885 (2018).
Jain, A. et al. Commentary: the Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 11 (2013).
Li, Y. et al. Core–shell nanostructured magnesium-based hydrogen storage materials: a critical review. Ind. Chem. Mater. 1, 282–298 (2023).
Gao, Y. et al. Amorphous dual-layer coating: enabling high Li-ion conductivity of non-sintered garnet-type solid electrolyte. Adv. Funct. Mater. 31, 10 (2021).
He, X., Zhu, Y. & Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017).
Chao, B. & Klebanoff, L. in Hydrogen Storage Technology: Materials and Applications (ed. Klebanoff, L.) chap. 5 (Taylor & Francis, 2012).
Orimo, S., Nakamori, Y., Eliseo, J. R., Züttel, A. & Jensen, C. M. Complex hydrides for hydrogen storage. Chem. Rev. 107, 4111–4132 (2007).
Grochala, W. & Edwards, P. P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 104, 1283–1316 (2004).
Bogdanović, B. et al. Investigation of hydrogen discharging and recharging processes of Ti-doped NaAlH4 by X-ray diffraction analysis (XRD) and solid-state NMR spectroscopy. J. Alloy. Compd. 350, 246–255 (2003).
Bogdanović, B. & Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloy. Compd. 253–254, 1–9 (1997).
Ren, Z.-H. et al. Single Ti atoms coupled with Ti–O clusters enable low temperature hydrogen cycling by sodium alanate. Rare Met. 43, 2671–2681 (2024).
Liu, W., Liu, P. & Mitlin, D. Tutorial review on structure – dendrite growth relations in metal battery anode supports. Chem. Soc. Rev. 49, 7284–7300 (2020).
Conder, K. & Kaldis, E. High accuracy volumetric determination of hydrogen in rare-earth hydrides. J. Less-Common Met. 146, 205–211 (1989).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Anisimov, V. I., Aryasetiawan, F. & Lichtenstein, A. I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: the LDA + U method. J. Phys. Condens. Matter. 9, 767–808 (1997).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Augustyn, V. et al. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 12, 518–522 (2013).
Binnewies, M. & Milke, E. Thermochemical Data of Elements and Compounds 2nd edn (Wiley, 2002).
Rowberg, A. J., Weston, L. & Van de Walle, C. G. Ion-transport engineering of alkaline-earth hydrides for hydride electrolyte applications. Chem. Mater. 30, 5878–5885 (2018).
Priyanga, G. S., Rajeswarapalanichamy, R. & Iyakutti, K. First principles study of structural, electronic, elastic and magnetic properties of cerium and praseodymium hydrogen system REHx (RE: Ce, Pr and x = 2, 3). J. Rare Earths 33, 289–303 (2015).
Kulikov, N. I. & Tugushev, V. V. An electronic band structure model for the metal-semiconductor transition in cerium-group hydrides. J. Less Common Met. 74, 227–236 (1980).
Acknowledgements
We thank R. Wu for TOF-SIMS support; Q. Jiang and Y. Zhao for HRTEM analysis; and C. Xue for S-XRD assistance. This work was jointly supported by the Liaoning Binhai Laboratory (grant no. LBLE-2023-01), the Science and Technology Major Project of Liaoning Province (grant no. 2024JH1/11700013), the National Natural Science Foundation of China (grant nos. 22309175, 21988101, 22279130 and 21633011), the Dalian Institute of Chemical Physics (DICP I202330) and the Dalian Science and Technology Innovation Fund (2023RJ016).
Author information
Authors and Affiliations
Contributions
P.C. conceived the project. J.C. synthesized the hydride ion conductor, P.C., J.C. and R.Z. designed the battery. J.C., R.Z., W.Z., S.W., S.L. and H. Chen. performed the structural characterization and electrochemical testing. H.W. and J. Liu. performed the DFT calculations. W.L., X.J. and W.W. conducted the HRTEM, NMR and S-XRD measurements, respectively. T.G. and J.Li. conducted the XAS measurements. P.C. and H. Cao. supervised the study. W.Z. repeated material synthesis, battery assembly and electrochemical testing. J.C., R.Z., W.Z., H.W., J. Liu., J.G., T.H., H. Cao. and P.C. discussed and analysed the results. J.C. and P.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Composition and structural properties of the as-prepared samples.
a, Hydrogen contents of the samples. *The hydrogen contents were determined by acidolysis of MHx using a hydrochloric acid solution. b, XRD patterns of the as-prepared BaH2 and CeH3. Black ticks, CeH3; red ticks, BaH2. c, XRD patterns of the nCeH3-BaH2 (n = 2, 3, 4, 5) samples. A certain amount (50% (V/V)) of silicon powder was added into the samples as an internal standard to accurately identify the phases of the mixtures. The main phase of all the nCeH3-BaH2 samples is the cubic CeH3 \(({Fm}\overline{3}m)\). The phase of BaH2 is not obvious even in the samples with higher BaH2 contents. d, XRD patterns of the 3CeH3-BaH2 samples ball milled for different time. As the milling time increases, the BaH2 phase weakens and disappears, while the crystalline phase of CeH3 retains. e, Diffuse reflectance infrared Fourier transform (DRIFT) spectra of BaH2, CeH3 and 3CeH3-BaH2 samples. The vibration bands centred at 1170 and 915 cm−1 are corresponding to Ce-H bonds and the bands centred at 1075 and 775 cm−1 belong to BaH2. After ball milling, the signals of BaH2 preserve. Noted that DRIFT is a surface sensitive technique. Although the molar ratio of BaH2 accounts only for 1/4 of the sample, the signal of Ba-H is relatively strong, indicating that BaH2 could be enriched on the surface of the material.
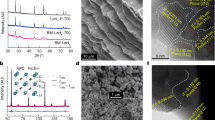
Extended Data Fig. 2 Morphology and structure characterizations.
a,b, SEM images of the ball milled 3CeH3-BaH2 and CeH3 samples, respectively. The 3CeH3-BaH2 sample has a larger particle size, mainly in the rage from 0.5 to 1 μm. The particle size of the ball milled CeH3 is smaller, mainly in 0.2 to 0.6 μm. c, SEM image and corresponding EDS mapping of the 3CeH3-BaH2 sample. The green and red dots represent cerium and barium, respectively. In the micrometre scale, Ba and Ce are uniformly distributed. d, HRTEM images of the 3CeH3-BaH2 sample. Red parallel lines mark the observed crystal plane spacings. These crystal plane data match well with CeH3 crystals \(({Fm}\overline{3}m)\), no crystallines related to BaH2 (Pnma) can been found. e, TEM image of the 3CeH3@BaH2 sample and its corresponding SAED pattern. The diffraction pattern corresponds to the CeH3 crystal, and the innermost ring belongs to the CeH3 (111) plane. No crystalline BaH2 was detectable (if there exists BaH2 crystal in the particle, the innermost ring radius of the diffraction pattern should be much smaller because the crystal plane spacing of orthorhombic BaH2 is larger than 0.32 nm). f, TEM and EELS mapping of the 3CeH3@BaH2 sample. The upper-left image shows the microscopic image of the particle edge, and the green box shows the scanning area. Other figures show the corresponding distribution of Ba and Ce elements. It is evident that particles with core-shell structures are clustered together.
Extended Data Fig. 3 Simulated structure and electronic properties of CeH3@BaH2 heterostructure.
a, Structure model of CeH3 (111)/BaH2 (010) heterojunction and the partial density of states (PDOS) of BaH2 (010) and CeH3 (111). The average bond lengths of Ba-H in BaH2, Ce-H in CeH3, and the Ba-H and Ce-H bonds at the interface are 2.77, 2.36, 2.61 and 2.49 Å, respectively. b, Charge density difference (Δρ) and planar-averaged along the z-axis for the CeH3-BaH2 heterojunctions, showing electron accumulation (yellow) and depletion (blue) across the interface. c, PDOS of the CeH3 (111) slab calculated with PBE + U, showing states near the Fermi level with a small band gap of ~0.12 eV. d, PDOS of the BaH2 (010) slab calculated with HSE06. This slab has a band gap of 3.27 eV that is slightly lower than the value (3.85 eV) of bulk material. These findings indicate an interfacial electron transfer from BaH2 to CeH3.
Extended Data Fig. 4 XPS and XANES spectra of various samples.
a, XPS spectra of the Ce 3 d for various samples. b, XPS spectra of the Ba 3d5/2 for various samples. Ba of the sample prepared by hand mixing (denoted by 3CeH3-BaH2-HM) and the neat BaH2 sample have a lower binding energy, while the binding energies of post-ball milled samples are higher. This implies that Ba atoms transfer more electrons to H atoms at the interface of the BaH2/CeH3 heterojunction of the ball milled sample. Little variation was observed for Ce among all the samples. c, Ce L3-edge XANES spectra of various samples. d, Ba L3-edge XANES spectra of various samples. Ba of 3CeH3@BaH2 has a higher peak intensity than neat BaH2 and 3CeH3-BaH2-HM samples, showing it is in a higher oxidative state. This is consistent with the XPS results. Ce, on the other hand, does not show remarked difference among the samples.
Extended Data Fig. 5 Electrochemical performance of 3CeH3@BaH2.
a, The EIS plots at 180, 200 and 220 °C. The inset is the EIS plot and the equivalent circuit of the 3CeH3@BaH2 at 20 °C. Rbulk, resistance of bulk; Rgb, resistance of grain boundary. b, σi, σe, and ti of the nCeH3-BaH2 samples with different ratios at 20 °C. The 3:1 sample has a high ionic conductivity and the highest transfer number. c, The LSV curve of the 3CeH3@BaH2 sample in H2 (black line) and Ar (blue line) atmospheres (1.0 bar) at room temperature. Its electrochemical window is around 1.0 V. d, Arrhenius plot for the ionic conductivity of the 3CeH3@BaH2. Diffusion barrier was derived from the fitted line.
Extended Data Fig. 6 Cycling tests of the LaH3 | 3CeH3@BaH2 | LaH3 symmetric cell.
The tests were conducted at current densities of 1.3 and 2.7 mA cm−2, respectively.
Extended Data Fig. 7 Electrochemical performance of the CeH2 | 3CeH3@BaH2 | NaAlH4 battery.
a, The rate capability of the hydride ion battery. Because of substantial capacity fading during the first few cycles, data collection commenced from the tenth cycle. b, Cyclic voltammetry (CV) curves of the CeH2 | 3CeH3@BaH2 | NaAlH4 battery at different sweep rates. There are obvious oxidation and reduction peaks in the CV curves, and the peak positions correspond to the highest rates of charge and discharge processes. The areas belonging to the positive and negative currents are nearly the same, indicating reversibility of the electrode reactions. c, Logarithm peak current (i) versus logarithm sweep rate (v) plots to determine the b-value of redox peaks. The b values of three redox peaks were calculated to be 0.610, 0.533, and 0.508, respectively, suggesting that this battery mainly follows a diffusion-dominated process36.
Extended Data Fig. 8 S-XRD patterns of the anode after discharge and charge.
The peaks of the anode (CeH2) shifted significantly to higher angles after discharge, indicating an increase in hydrogen content in Ce hydride; after recharge, asymmetric peaks with a trend of moving towards smaller angles were observed, indicating a decrease in hydrogen content.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, J., Zou, R., Zhang, W. et al. A room temperature rechargeable all-solid-state hydride ion battery. Nature 646, 338–342 (2025). https://doi.org/10.1038/s41586-025-09561-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09561-3