Abstract
The evolution of a single-dentary-boned lower jaw and its secondary craniomandibular articulation between the dentary condyle and the squamosal glenoid has been regarded as a pivotal vertebrate innovation and defining mammalian trait1,2,3,4,5,6,7. Here we report two mammaliamorphs with novel shapes of secondary jaw joint, offering insight into the evolution of the mammalian jaw. The first, Polistodon8, a Middle Jurassic herbivorous tritylodontid with a relatively large body size and a lifestyle that is likely to have been fossorial, uniquely evolved a dentary–jugal articulation. The second, an Early Jurassic morganucodontan, exhibits a dentary–squamosal joint that lacks a bulbous condyle, supporting the hypothesis that the mammalian dentary condyle was formed by expansion of the lateral ridge of the dentary9. These diverse joints reflect repeated evolutionary experimentation in advanced cynodonts, in which secondary jaw joints arose independently7,10, and in which the load-bearing dentary–squamosal joint is a synapomorphy of mammaliaforms. Although body miniaturization might have driven this transformation11, our findings indicate that other factors were involved, such as jaw-muscle reorganization, feeding ecology and masticatory behaviour7,12,13,14,15,16,17. The ecomorphological diversity of these taxa suggest that phenotypic plasticity and environmentally induced morphological changes18,19,20 could have shaped jaw-joint evolution, emphasizing how ecological pressures and developmental flexibility guided the diversification of jaw structures in mammalian ancestors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All material related to the data for phylogenetic analysis is presented in this Article and its Supplementary Information. Life science identifiers for the new genus and species have been registered at ZooBank: article: 278B9AE4-94E2-4003-ACFE-5C6497C7514E; Polistodon: 6B85D440-D84E-4A14-B952-28A1D60B016B; Polistodon chuannanensis: 2176D79D-3A6C-4293-94F1-3684488EA7B7; Camurocondylus: 454DF3EF-A9E7-4C62-9987-BB551CE54F64; Camurocondylus lufengensis: FC798AB8-CF78-498D-8B34-745EBC496CAD. The character list and data matrix for the phylogenetic analysis are presented in the Supplementary Information and have been deposited in MorphoBank (http://morphobank.org/permalink/?P5985). The 3D stl files for the skulls of Polistodon and Camurocondylus have been uploaded to the Archives of Digital Morphology, Key Laboratory of Vertebrate Evolutionary Systems Science of the Chinese Academy of Sciences (http://www.admorph.ivpp.ac.cn): Polistodon: https://doi.org/10.12112/M.36.Fossil; Camurocondylus: https://doi.org/10.12112/M.38.Fossil.
Code availability
The PAUP commands for parsimony-based analysis are in the Supplementary Information. The character list and data matrix for the phylogenetic analysis are in the Supplementary Information and have also been deposited in Zenodo (https://doi.org/10.5281/zenodo.16193266; ref. 72).
References
Crompton, A. The cranial morphology of a new genus and species of ictidosauran. Proc. Zool. Soc. Lond. 130, 183–216 (1958).
Kermack, K. A. & Mussett, F. The jaw articulation of the Docodonta and the classification of Mesozoic mammals. Proc. R. Soc. Lond. B 149, 204–215 (1958).
Simpson, G. G. Diagnosis of the classes Reptilia and Mammalia. Evolution 14, 388–392 (1960).
Barghusen, H. R. & Hopson, J. A. Dentary–squamosal joint and the origin of mammals. Science 168, 573–575 (1970).
Rowe, T. Definition, diagnosis, and origin of Mammalia. J. Vertebr. Paleontol. 8, 241–264 (1988).
Luo, Z.-X., Kielan-Jaworowska, Z. & Cifelli, R. L. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontol. Pol. 47, 1–78 (2002).
Rawson, J. R. et al. Brazilian fossils reveal homoplasy in the oldest mammalian jaw joint. Nature 634, 381–388 (2024).
He, X. & Cai, K. The tritylodont remains from Dashanpu, Zigong. J. Chengdu Coll. Geol. Suppl. 2, 33–45 (1984).
Crompton A, & Parkyn, D. On the lower jaw of Diarthrognathus and the origin of the mammalian lower jaw. Proc. Zool. Soc. Lond. 140, 697–749 (1963).
Reed, D., Iriarte‐Diaz, J. & Diekwisch, T. A three dimensional free body analysis describing variation in the musculoskeletal configuration of the cynodont lower jaw. Evol. Dev. 18, 41–53 (2016).
Lautenschlager, S., Gill, P. G., Luo, Z.-X., Fagan, M. J. & Rayfield, E. J. The role of miniaturization in the evolution of the mammalian jaw and middle ear. Nature 561, 533–537 (2018).
Crompton, A. W. The evolution of the mammalian jaw. Evolution 17, 431–439 (1963).
Kermack, K. A., Mussett, F. & Rigney, H. W. The lower jaw of Morganucodon. Zool. J. Linn. Soc. 53, 87–175 (1973).
Bramble, D. M. Origin of the mammalian feeding complex: models and mechanisms. Paleobiology 4, 271–301 (1978).
Sues, H.-D. Skull and dentition of two tritylodontid synapsids from the Lower Jurassic of western North America. Bull. Mus. Comp. Zool. 151, 217–268 (1986).
Crompton, A. W. & Hylander, W. in The Ecology and Biology of Mammal-like Reptiles (eds Nicholas, H. et al.) 263–282 (1986).
Grossnickle, D. M., Weaver, L. N., Jäger, K. R. & Schultz, J. A. The evolution of anteriorly directed molar occlusion in mammals. Zool. J. Linn. Soc. 194, 349–365 (2022).
West-Eberhard, M. Developmental Plasticity and Evolution (Oxford Univ. Press, 2003).
Gilbert, S. F., Bosch, T. C. & Ledón-Rettig, C. Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 16, 611–622 (2015).
Müller, G. B. Why an extended evolutionary synthesis is necessary. Interface Focus 7, 20170015 (2017).
Crompton, A. W. in Studies in Vertebrate Evolution (eds Joysey, K. A. & Kemp, T. S.) 231–251 (Oliver & Boyd, 1972).
Kemp, T. S. The Origin and Evolution of Mammals (Oxford Univ. Press, 2005).
Kermack, K. A. The interrelations of early mammals. Zool. J. Linn. Soc. 47, 241–249 (1967).
Gow, C. The dentitions of the Tritheledontidae (Therapsida: Cynodontia). Proc. R. Soc. Lond. B 208, 461–481 (1980).
Allin, E. F. & Hopson, J. A. in The Evolutionary Biology of Hearing (eds Webster, D. B. et al.) 587–614 (Springer, 1992).
Patterson, B. & Olson, E. C. A triconodontid mammal from the Triassic of Yunnan. In International Colloquium On The Evolution Of Lower And Non-specialized Mammals 129–191 (Koninklijke Vlaamse Academiie voor Wetenschappen, 1961).
Kermack, K. A., Mussett, F. & Rigney, H. W. The skull of Morganucodon. Zool. J. Linn. Soc. 71, 1–158 (1981).
Mao, F. et al. Fossils document evolutionary changes of jaw joint to mammalian middle ear. Nature 628, 576–581 (2024).
Fourie, S. The jaw articulation of Tritylodontoideus maximus. S. Afr. J. Sci. 64, 255–265 (1968).
Crompton, A. & Sun, A.-L. Cranial structure and relationships of the Liassic mammal Sinoconodon. Zool. J. Linn. Soc. 85, 99–119 (1985).
Crompton, A. W. in Functional Morphology in Vertebrate Paleontology (ed. Thomason, T.) 55–75 (Cambridge Univ. Press, 1995).
Jasinoski, S. C. & Chinsamy, A. Mandibular histology and growth of the nonmammaliaform cynodont Tritylodon. J. Anat. 220, 564–579 (2012).
Allin, E. F. Evolution of the mammalian middle ear. J. Morphol. 147, 403–437 (1975).
Sidor, C. A. Evolutionary trends and the origin of the mammalian lower jaw. Paleobiology 29, 605–640 (2003).
Bertossa, R. C. Morphology and behaviour: functional links in development and evolution. Philos. Trans. R. Soc. B 366, 2056–2068 (2011).
Newman, S. A. & Müller, G. B. Epigenetic mechanisms of character origination. J. Exp. Zool. 288, 304–317 (2000).
Clark, J. M. & Hopson, J. A. Distinctive mammal-like reptile from Mexico and its bearing on the phylogeny of the Tritylodontidae. Nature 315, 398–400 (1985).
Lopatin, A. & Agadjanian, A. A tritylodont (Tritylodontidae, Synapsida) from the Mesozoic of Yakutia. Dokl. Biol. Sci. 419, 279–282 (2008).
Matsuoka, H., Kusuhashi, N. & Corfe, I. J. A new Early Cretaceous tritylodontid (Synapsida, Cynodontia, Mammaliamorpha) from the Kuwajima Formation (Tetori Group) of central Japan. J. Vertebr. Paleontol. 36, e1112289 (2016).
Averianov, A. O. et al. A tritylodontid synapsid from the Middle Jurassic of Siberia and the taxonomy of derived tritylodontids. J. Vertebr. Paleontol. 37, e1363767 (2017).
Velazco, P. M., Buczek, A. J. & Novacek, M. J. Two new tritylodontids (Synapsida, Cynodontia, Mammaliamorpha) from the Upper Jurassic, southwestern Mongolia. Am. Mus. Novit. 2017, 3874 (2017).
Panciroli, E., Walsh, S., Fraser, N. C., Brusatte, S. L. & Corfe, I. A reassessment of the postcanine dentition and systematics of the tritylodontid Stereognathus (Cynodontia, Tritylodontidae, Mammaliamorpha), from the Middle Jurassic of the United Kingdom. J. Vertebr. Paleontol. 37, e1351448 (2017).
Mao, F., Zhang, C., Liu, C. & Meng, J. Fossoriality and evolutionary development in two Cretaceous mammaliamorphs. Nature 592, 577–582 (2021).
Sun, A. L. Skull morphology of the tritylodont genus Bienotheroides of Sichuan. Sci. Sin. B 27, 970–984 (1984).
Liu, L. et al. New discovery of tritylodontids from the Middle Jurassic in Yunyang area, Chongqing and their paleogeographic significance. J. Palaeogeogr. 26, 1–15 (2024).
Crompton, A. W. & Jenkins, F. A. Molar occlusion in Late Triassic mammals. Biol. Rev. 43, 427–458 (1968).
Crompton, A. W. Postcanine occlusion in cynodonts and tritylodontids. Bull. Br. Mus. (Nat. Hist.) 21, 27–71 (1972).
Kalthoff, D. C. et al. Complementary approaches to tooth wear analysis in Tritylodontidae (Synapsida, Mammaliamorpha) reveal a generalist diet. PLoS One 14, e0220188 (2019).
Hopkins, S. S. The evolution of fossoriality and the adaptive role of horns in the Mylagaulidae (Mammalia: Rodentia). Proc. R. Soc. B 272, 1705–1713 (2005).
Liu, J., Soares, M. B. & Reichel, M. Massetognathus (Cynodontia, Traversodontidae) from the Santa Maria Formation of Brazil. Rev. Bras. Paleontol. 11, 27–36 (2008).
Hopson, J. A. & Kitching, J. W. A revised classification of cynodonts (Reptilia; Therapsida). Palaeontol. Afr. 14, 71–85 (1972).
Hopson, J. & Crompton, A. in Evolutionary Biology (eds Dobzhansky, T. et al.) 16–72 (Appleton-Century-Crofts, 1969).
Anthwal, N. & Tucker, A. S. Evolution and development of the mammalian jaw joint: Making a novel structure. Evol. Dev. 25, 3–14 (2023).
Hunter, J. P. & Jernvall, J. The hypocone as a key innovation in mammalian evolution. Proc. Natl Acad. Sci. USA 92, 10718–10722 (1995).
Donoghue, M. J. & Sanderson, M. J. Confluence, synnovation, and depauperons in plant diversification. New Phytol. 207, 260–274 (2015).
Erwin, D. H. A conceptual framework of evolutionary novelty and innovation. Biol. Rev. 96, 1–15 (2021).
Müller, G. B. & Wagner, G. P. Novelty in evolution: restructuring the concept. Annu. Rev. Ecol. Syst. 22, 229–256 (1991).
Arthur, W. Intraspecific variation in developmental characters: the origin of evolutionary novelties. Am. Zool. 40, 811–818 (2000).
Martin, T. & Rauhut, O. W. M. Mandible and dentition of Asfaltomylos patagonicus (Australosphenida, Mammalia) and the evolution of tribosphenic teeth. J. Vertebr. Paleontol. 25, 414–425 (2005).
Morales-García, N. M., Gill, P. G., Janis, C. M. & Rayfield, E. J. Jaw shape and mechanical advantage are indicative of diet in Mesozoic mammals. Commun. Biol. 4, 242 (2021).
Debuysschere, M., Gheerbrant, E. & Allain, R. Earliest known European mammals: a review of the morganucodonta from Saint-Nicolas-de-Port (Upper Triassic, France). J. Syst. Palaeontol. 13, 825–855 (2015).
Gill, P. G. et al. Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature 512, 303–305 (2014).
Sues, H.-D. The relationships of the Tritylodontidae (Synapsida). Zool. J. Linn. Soc. 85, 205–217 (1985).
Cooper, K. L. The case against simplistic genetic explanations of evolution. Development 151, dev203077 (2024).
van Valen, L. Festschrift: Evolutionary Biology. Vol. 6. Theodosius Dobzhansky, Max K. Hecht and William C. Steere, eds. Science 180, 488 (1973).
Simpson, G. G. A Catalogue of the Mesozoic Mammalia in the Geological Department of the British Museum (Trustees of the British Museum, 1928).
Setoguchi, T., Matsuda, M. & Matsuoka, H. New discovery of an Early Cretaceous tritylodontid (Reptilia, Therapsida) from Japan and the phylogenetic reconstruction of Tritylodontidae based on the dental characters. In Seventh Annual Meeting of the Chinese Society of Vertebrate Paleontology (eds Wang Y. Q. & Deng, T.) 117-124 (China Ocean Press, 1999).
Matsuoka, H. in Fossils of the Kuwajima “Kaseki-kabe” (Fossil-bluff): Scientific Report on a Neocomian (Early Cretaceous) Fossil Assemblage from the Kuwajima Formation, Tetori Group, Shiramine, Ishikawa, Japan (ed. Matsuoka, H.) 53–74 (Shiramine Village Board of Education, 2000).
Watabe, M., Tsubamoto, T. & Tsogtbaatar, K. A new tritylodontid synapsid from Mongolia. Acta Palaeontol. Pol. 52, 263–274 (2007).
Davis, B. M., Jäger, K. R. K., Rougier, G. W., Trujillo, K. & Chamberlain, K. A morganucodontan mammaliaform from the Upper Jurassic Morrison Formation, Utah, USA. Acta Palaeontol. Pol. 67, 77–93 (2022).
Mao, F. et al. Jurassic shuotheriids show earliest dental diversification of mammaliaforms. Nature 628, 569–575 (2024).
Mao, F. et al. Convergent evolution of diverse jaw-joints in mammaliamorph. Zenodo https://doi.org/10.5281/zenodo.16193266 (2025).
Luo, Z.-X. Developmental patterns in Mesozoic evolution of mammal ears. Annu. Rev. Ecol. Syst. 42, 355–380 (2011).
Soares, M. B., Schultz, C. L. & Horn, B. L. New information on Riograndia guaibensis Bonaparte, Ferigolo & Ribeiro, 2001 (Eucynodontia, Tritheledontidae) from the Late Triassic of southern Brazil: anatomical and biostratigraphic implications. An. Acad. Bras. Cienc. 83, 329–354 (2011).
Rodrigues, P. G. et al. Digital cranial endocast of Riograndia guaibensis (Late Triassic, Brazil) sheds light on the evolution of the brain in non-mammalian cynodonts. Hist. Biol. 31, 1195–1212 (2019).
Kerber, L. et al. An additional brain endocast of the ictidosaur Riograndia guaibensis (Eucynodontia: Probainognathia): intraspecific variation of endocranial traits. An. Acad. Bras. Cienc. 93, e20200084 (2021).
Mao, F. & Meng, J. A new haramiyidan mammal from the Jurassic Yanliao Biota and comparisons with other haramiyidans. Zool. J. Linn. Soc. 186, 529–552 (2019).
Meng, J., Wang, Y. & Li, C. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature 472, 181–185 (2011).
Mao, F. et al. Integrated hearing and chewing modules decoupled in a Cretaceous stem therian mammal. Science 367, 305–308 (2020).
Mao, F., Liu, C., Chase, M. H., Smith, A. K. & Meng, J. Exploring ancestral phenotypes and evolutionary development of the mammalian middle ear based on Early Cretaceous Jehol mammals. Natl Sci. Rev. 8, nwaa188 (2021).
Acknowledgements
We thank G. Peng and G. Wei for access to the specimens; Y. Hou, P. Yin and J. Wang for CT scanning of the specimens; A. Shi for help with drawings; J. Choiniere and J. Benoid for access to collections; and S. Lautenschlager and DigiMorph.org for digital data. F.M. was supported by the National Natural Science Foundation of China (42122010 and 42288201) and by the Youth Innovation Promotion Association of the Chinese Academy of Sciences (Y2023017). S.J., Y.Y., Y.L. and X.S. were supported by the Project Foundation of the Sichuan Provincial Cultural Heritage Administration (SCWW2023A02). P.W. and J.C. were supported by the Chongqing Municipal Planning and Natural Resource Bureau (ZC-2021018).
Author information
Authors and Affiliations
Contributions
F.M. and J.M. conceived the study and wrote the paper. F.M. and J.R. performed the CT scanning and rendering work. S.J., Y.Y., Y.L., X.S., T.W., G.W., P.W. and J.C. participated in the fieldwork and provided stratigraphic data. J.L. helped with refining the data matrix, and provided discussions and manuscript edits. All authors edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Neal Anthwal, Julia Schultz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
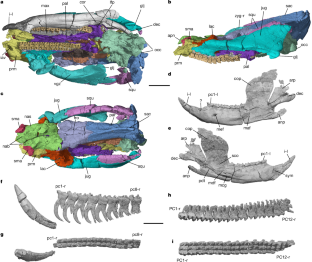
Extended Data Fig. 1 Holotype of Polistodon chuannanensis (ZDM8601).
a, Dorsal view with the lower jaws in occlusion; b, Lateral (left); c, ventral; d, lateral (right); e, anterior; f, posterior; g, ventrolateral (right). Abbreviations: anp, angular process; apn, anterior process of the nasal; cop, coronoid process; dec, dentary condyle; dja, dentary–jugal articulation; glj, glenoid of the jugal; iav, incisor alveolus; i, incisor; jug, jugal; lac, lacrimal; nab, nasal boss; occ, occipital condyle; pdt, postdentary trough; sac, sagittal crest; sjs, squamosal-jugal suture; sma, septomaxilla; squ, squamosal; sym, symphysis; vgz, ventral groove of the zygma (of jugal).
Extended Data Fig. 2 Digital rendered holotype of Polistodon chuannanensis (ZDM8601).
a, Ventral view of the skull with both lower jaws in occlusion. b, Lateral (right) view of the cranium; c, lateral (right) view of the squamosal, jugal, and the mandible; d, lateral (right) view of the mandible (same position in c); e, posterior view of the squamosal and jugal; f, posterior view of the squamosal, jugal, and the mandible from both sides; g, posteromedial view of the right squamosal, jugal and mandible with the coronoid attached to the dentary; h, posterior view of the skull with the left mandible digitally removed. i–k, Ventral (i), dorsal (j), and posterior (k) views of the two lower jaws. Abbreviations: anp, angular process; cop, coronoid process; cor, coronoid; dec, dentary condyle; dja, dentary–jugal articulation; dpg, dorsal process of the glenoid; glj, glenoid of the jugal; i, incisor; jug, jugal; lac, lacrimal; loj, lower jaw(s); maf, masseteric fossa; mef, mental foramen; nas, nasal; occ, occipital condyle; ocp, occipital plate (bones fused in the occiput); par, parietal; prm, premaxilla; sjs, squamosal-jugal suture; sma, septomaxilla; squ, squamosal; sym, symphysis; vgz, ventral groove of the zygma (of jugal); vpg, ventral process of the glenoid.
Extended Data Fig. 3 Digital rendered postcanines of Polistodon chuannanensis (holotype, ZDM8601).
a–c, Lateral (right, but medial for left dentition) (a), occlusal (b), and lateral (left, right medial for right dentition) (c) views of the two lower dentitions as preserved. d–g, Lingual (d), occlusal (e), dorsal (f), and lateral (g) views of the right upper postcanines. h, Occlusal view of the left upper postcanines. i,j, Lateral (i) and lingual (j) views the left dentary (digitally rendered as semitransparent) and teeth. Abbreviations: -r, right side; -l, left side; anr, anterior root; arp, articular process; cop, coronoid process; dec, dentary condyle; i, incisor; pc, lower postcanine; PC, upper postcanine; por, posterior root.
Extended Data Fig. 4 Comparison of the jaw system of P. chuannanensis (holotype, ZDM8601) with the typical tritylodontid pattern represented by Bienotheroides.
a–c, Ventral view of the cranium of P. chuannanensis (a) and Bienotheroides (b, 16YPDC-01; c, 19YP-67). d–f, Lateral views of the same specimens in a–c. g–i, Medial view of the zygomatic arch formed by the jugal and squamosal, corresponding to a–c, respectively. j–l, Medial (j), posteromedial (k), and lateral (l) views of the dentary of P. chuannanensis. m–o, Medial (m), posteromedial (n), and lateral (o) views of the dentary of Bienotheroides (20YX-1#-0510-1)45. Abbreviations: ang, angular process; arp, articular process; bwp, bulged wall of the postdentary trough; cop, coronoid process; dec, dentary condyle; dpg, dorsal process of the glenoid; glj, glenoid of the jugal; i, incisor; jug, jugal; maf, masseteric fossa; mcg, Mecklian groove; mef, mental foramen; mer, medial ridge; pdt, postdentary trough; sjs, squamosal-jugal suture; squ, squamosal; vgz, ventral groove of the zygoma (of jugal); vpg, ventral process of the glenoid.
Extended Data Fig. 5 Holotype specimen of Camurocondylus lufengensis gen. et sp. nov. (IVPP V8685).
a–c, Lateral (right and left) (a,b) and ventral (c) views of the skull as preserved (a,b) and after the mandibles were removed (c). d, Computed tomographic section showing the tooth root condition in upper and lower jaws. e,f, Ventral (e) and dorsal (f) views of the skull with the bones segmented (in colour). g,h, Lateral (g) and lateroventral (h) views of the right upper and lower dentitions in preserved occlusal relation.
Extended Data Fig. 6 Dentitions of Camurocondylus lufengensis gen. et sp. nov. (holotype, IVPP V8685).
a–c, Occlusal (a), labial (b) and lingual (c) views of the left ultimate upper premolar and M1-3. d–f, Occlusal (d), lingual (e) and labial (f) views of the right ultimate premolar and M1-3. g–i, Occlusal (g), labial (h) and lingual (i) views of left m1-3. j–l, Occlusal (j), lingual (k), and labial (l) views of the right ultimate premolar and m1-3. Tooth measurements (length/width in mm; asterisk denoting estimate): left pc (px 1.1*/0.69*; m1 2.29/0.84; m2 2.45/1.84; m3 1.95/1.04); left PC (P1 0.44*/0.23*; P2 0.63*/0.39; P3 1.89/0.72; M1 2.04/1.85; M2 1.98/0.96; M3 1.13/0.69); right pc (px 1.8*/0.82; m1 2.33/0.97; m2 2.41/1.21; m3 1.93/0.93); right PC (P1 p.45*/0.24*; P2 0.55*/0.32*; P3 1.79/0.78; M1 2.05/0.91; M2 1.95/1.0; M3 1.1/0.77).
Extended Data Fig. 7 Comparison of load-bearing dentary condyles in mammaliaforms.
a–j, The dentary condyles of various extinct groups (a-c, f-j, corresponding to the taxon names at the bottom of the panels) in comparison with those of extant mammals (d,e). In each panel, the dorsal, lateral, and posterior views of the condyle are arranged from the top to the bottom. To facilitate comparison some images have been photographically reversed so that the views are consistent throughout. All images are original except for Morganucodon, which is from Kermack et al.13 used with permission. The images are not at the same scale. Abbreviations: ang, angular process; ann, angular notch; cop, coronoid process; dec, dentary condyle; lpc, lateral projection of the condyle; ltr, lateral ridge; maf, masseteric fossa; mpc, medial projection of the condyle.
Extended Data Fig. 8 Images of jaw joints rendered from CT scans.
Images show jaw joints that are used as the basis for Fig. 4. a, Thrinaxodon (AMNH 5630), exclusive articular–quadrate joint. b, Probainognathus11, articular–quadrate joint with surangular–squamosal contact. Interpretation of the jaw joint of Thrinaxodon and Probainognathus in Fig. 4 also refers to Allin and Hopson25 (Fig. 28.8) and Luo73 (Fig. 3). c, Riograndia7 articular–quadrate joint with the dentary–squamosal contact. Interpretation of its jaw joint also refers to Soares et al.74, Rodrigues et al.75, and Kerber et al.76. d, Polistodon (ZDM8601), dentary–jugal joint; its primary joint in Fig. 4 is reconstructed based on tritylodontid specimen (IVPP V14232). e, Dianoconodon28 (IVPP V4257), partial but load-bearing dentary–squamosal jaw joint (PDSJ) with a bulbous dentary condyle and coexisting with a reduced but functional articular–quadrate joint. f, Camurocondylus (IVPP V8685), PDSJ with no expanded dentary condyle; its primary joint in Fig. 4 is reconstructed based on other morganucodontans, including Dianoconodon. g, Feredocodon71 (IMMNH-PV01925), exclusive dentary–squamosal joint (EDSJ) with postdentary bones remaining long and attached to the dentary but the articular–quadrate joint has completely lost its jaw suspension function. h, Qishou77 (JZT-D061), EDSJ for palinal jaw movement. i, Liaoconodon78,79,80 (IVPP V16051), EDSJ with the ossified Mechel’s cartilage (ONC) bridging the dentary and the detached postdentary bones (middle ear ossicles) in which the anterior processes of the malleus and ectotympanic are long and have substantial overlap with the OMC. j, Origolestes79,80 (JZD-DB0064), EDSJ with a long OMC that is decoupled from the middle ear ossicles in which the anterior processes of the malleus and ectotympanic are reduced. k, Tachyglossus aculeatus (AMNH teaching specimens, no catalogue number), EDSJ highly specialized and teeth are lost. l, Didelphis (AMNH 137900), EDSJ with medial extension of the dentary condyle, the middle ear ossicles fully suspended at the basicranial region, and a horseshoe shaped ectotympanic. m, Erinaceus (AMNH 57218), EDSJ with the ectotympanic expanded to from the tympanic bulla. All images are original except for b (3D rendering courtesy of Stephan Lautenschlager and DigiMorph.org; https://www.digimorph.org/index.phtml) and c (open-access publication).
Extended Data Fig. 9 Strict consensus tree of 211 best trees.
Tree length = 3,026; consistency index (CI) = 0.3275; homoplasy index (HI) = 0.6725; retention index (RI) = 0.8079; rescaled consistency index (RC) = 0.2646. See Supplementary Information for additional information. The blue polygon represents the load-bearing dentary–squamosal jaw joint that diagnoses Mammaliaformes (in blue shape), including mammals. Red arrows point to the phylogenetic positions of the two taxa reported in the study. Red dots indicate taxa that are used in Fig. 4 in the main text that possess the secondary jaw joint except for Thrinaxodon that has only the exclusive articular–quadrate joint. See Supplementary Information for the result of the bootstrap analysis.
Supplementary information
Supplementary Information
Supplementary Information Part I provides a category summarizing different types of the primary and secondary jaw joints in known mammaliamorphs and their close relatives. In the Systematic Palaeontology, we provide the differentiate diagnoses (the amended diagnosis is in the main text) for Polistodon channanensis He and Cai, 1984 and a redescription of the holotype specimen (ZDM8601). Illustrations of ZDM8601 are in the main text and Extended Data figures. We also provide the differentiate diagnoses for the new morganucodontan genus and species Camurocondylus lufengensis. gen. et sp. nov. (the diagnosis is in the main text) and a detailed description of the holotype specimen (IVPP V8685). Supplementary Information Part I also has the character list that includes specific coding for three relevant genera, Polistodon, Camurocondylus and Diarthrognathus, in addition to other taxa used in a previous study (Mao et al., 2024a). The character list contains brief explanations about the characters and character coding that are modified based on the new data recognized in this study. Supplementary Information Part II includes the data matrix, PAUP phylogenetic analyses and the resulting logs of the analysis about the strict consensus tree. The related character list, dataset, settings and logs of these analyses are presented deposited at MorphoBank (http://www.morphobank.org) and Zenodo (https://doi.org/10.5281/zenodo.10597270) (See also Methods in the main text). Supplementary Information Part III is the online link to the 3D stl files of reconstructed skulls of the holotype of Polistodon channanensis (ZDM8601) and the holotype of Camurocondylus lufengensis. gen. et sp. nov. (IVPP V8685).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, F., Jiang, S., Liu, J. et al. Convergent evolution of diverse jaw joints in mammaliamorphs. Nature (2025). https://doi.org/10.1038/s41586-025-09572-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-09572-0