Abstract
Understanding how viral and host factors interact and how perturbations impact infection is the basis for designing antiviral interventions. Here we define the functional contribution of each viral and host factor involved in human cytomegalovirus infection in primary human fibroblasts through pooled CRISPR interference and nuclease screening. To determine how genetic perturbation of critical host and viral factors alters the timing, course and progression of infection, we applied Perturb-seq to record the transcriptomes of tens of thousands of CRISPR-modified single cells and found that, normally, most cells follow a stereotypical transcriptional trajectory. Perturbing critical host factors does not change the stereotypical transcriptional trajectory per se but can stall, delay or accelerate progression along the trajectory, allowing one to pinpoint the stage of infection at which host factors act. Conversely, perturbation of viral factors can create distinct, abortive trajectories. Our results reveal the roles of host and viral factors and provide a roadmap for the dissection of host–pathogen interactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw and processed sequencing data from all pooled screens and single-cell experiments were uploaded to the Gene Expression Omnibus (GSE165291).
Code availability
We used published software for pooled screen data processing (https://github.com/mhorlbeck/ScreenProcessing) and for the analysis of Perturb-seq data (https://github.com/thomasmaxwellnorman/perturbseq_demo) with modifications.
References
Mocarski Jr., E. S., Schenk, T., Griffiths, P. D. & Pass, R. F. Cytomegaloviruses. in Fields Virology 1960–2014 (Lippincott Williams & Wilkins, 2013).
Gatherer, D. et al. High-resolution human cytomegalovirus transcriptome. Proc. Natl Acad. Sci. USA 108, 19755–19760 (2011).
Marcinowski, L. et al. Real-time transcriptional profiling of cellular and viral gene expression during lytic cytomegalovirus infection. PLoS Pathog. 8, e1002908 (2012).
Stern-Ginossar, N. et al. Decoding human cytomegalovirus. Science 338, 1088–1093 (2012).
Weekes, M. P. et al. Quantitative temporal viromics: an approach to investigate host-pathogen interaction. Cell 157, 1460–1472 (2014).
Tirosh, O. et al. The transcription and translation landscapes during human cytomegalovirus infection reveal novel host-pathogen interactions. PLoS Pathog. 11, e1005288 (2015).
Jean Beltran, P. M., Mathias, R. A. & Cristea, I. M. A portrait of the human organelle proteome in space and time during cytomegalovirus infection. Cell Syst. 3, 361–373 (2016).
Sinzger, C. et al. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76, 741–750 (1995).
Yu, D., Silva, M. C. & Shenk, T. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl Acad. Sci. USA 100, 12396–12401 (2003).
Dunn, W. et al. Functional profiling of a human cytomegalovirus genome. Proc. Natl Acad. Sci. USA 100, 14223–14228 (2003).
Puschnik, A. S., Majzoub, K., Ooi, Y. S. & Carette, J. E. A CRISPR toolbox to study virus-host interactions. Nat. Rev. Microbiol. 15, 351–364 (2017).
Russell, A. B., Trapnell, C. & Bloom, J. D. Extreme heterogeneity of influenza virus infection in single cells. eLife 7, e32303 (2018).
Erhard, F. et al. scSLAM-seq reveals core features of transcription dynamics in single cells. Nature 571, 419–423 (2019).
van Diemen, F. R. et al. CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog. 12, e1005701 (2016).
Van Damme, E. & Van Loock, M. Functional annotation of human cytomegalovirus gene products: an update. Front. Microbiol. 5, 218 (2014).
McCormick, A. L., Skaletskaya, A., Barry, P. A., Mocarski, E. S. & Goldmacher, V. S. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316, 221–233 (2003).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Horlbeck, M. A. et al. Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. eLife 5, e19760 (2016).
Compton, T., Nowlin, D. M. & Cooper, N. R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193, 834–841 (1993).
Hetzenecker, S., Helenius, A. & Krzyzaniak, M. A. HCMV induces macropinocytosis for host cell entry in fibroblasts. Traffic 17, 351–368 (2016).
Pusapati, G. V., Luchetti, G. & Pfeffer, S. R. Ric1-Rgp1 complex is a guanine nucleotide exchange factor for the late Golgi Rab6A GTPase and an effector of the medial Golgi Rab33B GTPase. J. Biol. Chem. 287, 42129–42137 (2012).
Smith, R. D. et al. The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin. Traffic 10, 1502–1517 (2009).
Liu, S., Dominska-Ngowe, M. & Dykxhoorn, D. M. Target silencing of components of the conserved oligomeric Golgi complex impairs HIV-1 replication. Virus Res. 192, 92–102 (2014).
Bassik, M. C. et al. A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell 152, 909–922 (2013).
Selyunin, A. S., Iles, L. R., Bartholomeusz, G. & Mukhopadhyay, S. Genome-wide siRNA screen identifies UNC50 as a regulator of Shiga toxin 2 trafficking. J. Cell Biol. 216, 3249–3262 (2017).
Starling, G. P. et al. Folliculin directs the formation of a Rab34-RILP complex to control the nutrient-dependent dynamic distribution of lysosomes. EMBO Rep. 17, 823–841 (2016).
Filipek, P. A. et al. LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning. J. Cell Biol. 216, 4199–4215 (2017).
Pu, J., Keren-Kaplan, T. & Bonifacino, J. S. A Ragulator-BORC interaction controls lysosome positioning in response to amino acid availability. J. Cell Biol. 216, 4183–4197 (2017).
Becker, T., Le-Trilling, V. T. K. & Trilling, M. Cellular cullin RING ubiquitin ligases: druggable host dependency factors of cytomegaloviruses. Int. J. Mol. Sci. 20, 163 (2019).
Le-Trilling, V. T. K. et al. Broad and potent antiviral activity of the NAE inhibitor MLN4924. Sci. Rep. 6, 19977 (2016).
Ott, M., Marques, D., Funk, C. & Bailer, S. M. Asna1/TRC40 that mediates membrane insertion of tail-anchored proteins is required for efficient release of herpes simplex virus 1 virions. Virol. J. 13, 175 (2016).
Nishitsuji, H., Ujino, S., Harada, K. & Shimotohno, K. TIP60 complex inhibits hepatitis B virus transcription. J. Virol. 92, e01788-17 (2018).
Kamine, J., Elangovan, B., Subramanian, T., Coleman, D. & Chinnadurai, G. Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-1 Tat transactivator. Virology 216, 357–366 (1996).
Adamson, B. et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167, 1867–1882 (2016).
Cohen, Y. et al. The yeast p5 type ATPase, spf1, regulates manganese transport into the endoplasmic reticulum. PLoS ONE 8, e85519 (2013).
Wu, K., Oberstein, A., Wang, W. & Shenk, T. Role of PDGF receptor-α during human cytomegalovirus entry into fibroblasts. Proc. Natl Acad. Sci. USA 115, E9889–E9898 (2018).
Soroceanu, L., Akhavan, A. & Cobbs, C. S. Platelet-derived growth factor-α receptor activation is required for human cytomegalovirus infection. Nature 455, 391–395 (2008).
Kabanova, A. et al. Platelet-derived growth factor-α receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat. Microbiol. 1, 16082 (2016).
Martinez-Martin, N. et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174, 1158–1171 (2018).
Tzelepis, K. et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in acute myeloid leukemia. Cell Rep. 17, 1193–1205 (2016).
Morgens, D. W., Deans, R. M., Li, A. & Bassik, M. C. Systematic comparison of CRISPR/Cas9 and RNAi screens for essential genes. Nat. Biotechnol. 34, 634–636 (2016).
Datlinger, P. et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Jaitin, D. A. et al. Dissecting immune circuits by linking CRISPR-pooled screens with single-cell RNA-seq. Cell 167, 1883–1896 (2016).
Zanini, F., Pu, S.-Y., Bekerman, E., Einav, S. & Quake, S. R. Single-cell transcriptional dynamics of flavivirus infection. eLife 7, e32942 (2018).
Drayman, N., Patel, P., Vistain, L. & Tay, S. HSV-1 single-cell analysis reveals the activation of anti-viral and developmental programs in distinct sub-populations. eLife 8, e46339 (2019).
Wyler, E. et al. Single-cell RNA-sequencing of herpes simplex virus 1-infected cells connects NRF2 activation to an antiviral program. Nat. Commun. 10, 4878 (2019).
Kotliar, D. et al. Single-cell profiling of Ebola virus disease in vivo reveals viral and host dynamics. Cell 183, 1383–1401 (2020).
An, P. et al. Single cell transcriptomics reveals a heterogeneous cellular response to BKV infection. J. Virol. 95, e02237 (2020).
Bogdanow, B. et al. Human cytomegalovirus tegument protein pp150 acts as a cyclin A2-CDK-dependent sensor of the host cell cycle and differentiation state. Proc. Natl Acad. Sci. USA 110, 17510–17515 (2013).
Spear, G. T. et al. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV). J. Immunol. 155, 4376–4381 (1995).
Isomura, H. & Stinski, M. F. Coordination of late gene transcription of human cytomegalovirus with viral DNA synthesis: recombinant viruses as potential therapeutic vaccine candidates. Expert Opin. Ther. Targets 17, 157–166 (2013).
Zhang, L., Yu, J. & Liu, Z. MicroRNAs expressed by human cytomegalovirus. Virol. J. 17, 34 (2020).
Winkler, M., Rice, S. A. & Stamminger, T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 68, 3943–3954 (1994).
Lischka, P., Toth, Z., Thomas, M., Mueller, R. & Stamminger, T. The UL69 transactivator protein of human cytomegalovirus interacts with DEXD/H-Box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Mol. Cell. Biol. 26, 1631–1643 (2006).
Schommartz, T., Tang, J., Brost, R. & Brune, W. Differential requirement of human cytomegalovirus UL112–113 protein isoforms for viral replication. J. Virol. 91, e00254-17 (2017).
Müller-Kuller, U. et al. A minimal ubiquitous chromatin opening element (UCOE) effectively prevents silencing of juxtaposed heterologous promoters by epigenetic remodeling in multipotent and pluripotent stem cells. Nucleic Acids Res. 43, 1577–1592 (2015).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Borst, E. M., Hahn, G., Koszinowski, U. H. & Messerle, M. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73, 8320–8329 (1999).
Adamson, B., Norman, T. M., Jost, M. & Weissman, J. S. Approaches to maximize sgRNA-barcode coupling in Perturb-seq screens. Preprint at bioRxiv https://doi.org/10.1101/298349 (2018).
Dunn, J. G. & Weissman, J. S. Plastid: nucleotide-resolution analysis of next-generation sequencing and genomics data. BMC Genomics 17, 958 (2016).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Ding, J., Shah, S. & Condon, A. densityCut: an efficient and versatile topological approach for automatic clustering of biological data. Bioinformatics 32, 2567–2576 (2016).
Heer, J., Kong, N. & Agrawala, M. Sizing the horizon: the effects of chart size and layering on the graphical perception of time series visualizations. in Proc. 27th Int. Conf. Human Factors in Computing Systems (ACM Press, 2009).
Murphy, E., Rigoutsos, I., Shibuya, T. & Shenk, T. E. Reevaluation of human cytomegalovirus coding potential. Proc. Natl Acad. Sci. USA 100, 13585–13590 (2003).
Acknowledgements
We thank M. A. Horlbeck for designing the HCMV tiling library; L. A. Gilbert for help setting up pooled screens; and T. M. Norman, M. A. Horlbeck, J. A. Hussmann and X. Qiu for help with data analysis. A. Xu, J. A. Villalta and R. A. Pak provided technical assistance. The UCOE sequence was a gift from G. Sienski. We thank T. Fair for help with Perturb-seq experiments. We thank N. Stern-Ginossar, M. J. Shurtleff, M. Jost, R. A. Saunders, J. M. Replogle, X. Qiu and all members of the Weissman lab for insightful discussions. J. Winkler and A. S. Puschnik provided helpful comments on the manuscript. Special thanks to O. Wueseke for editorial help. J.S.W. is a Howard Hughes Medical Institute Investigator. M.Y.H. was supported by an EMBO long-term postdoctoral fellowship (EMBO ALTF 1193-2015, co-funded by the European Commission FP7, Marie Curie Actions, LTFCOFUND2013, GA-2013-609409).
Author information
Authors and Affiliations
Contributions
M.Y.H. and J.S.W. conceptualized the study, interpreted the experiments and wrote the manuscript. M.Y.H. designed and carried out the experiments and analyzed the data.
Corresponding authors
Ethics declarations
Competing interests
J.S.W. has filed patent applications related to CRISPRi screening and Perturb-seq. J.S.W. consults for and holds equity in KSQ Therapeutics and Maze Therapeutics and consults for 5AM Ventures.
Additional information
Peer review information Nature Biotechnology thanks Antoine-Emmanuel Saliba and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
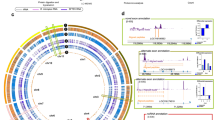
Extended Data Fig. 1 High-resolution HCMV tiling screen.
a, Data processing for the HCMV tiling screen. We calculated log2 ratios of each individual sgRNA in the surviving over the t0 populations, averaged across two biological replicates. Ratios were averaged in a sliding 250 bp window. The average of the ratios of the non-targeting sgRNA population was set as the baseline. The plot was then colored based on the sign of the average phenotype and layered in bands of decreasing lightness, one log2 unit wide. The negative space was mirrored on the baseline, and bands were stacked for the final horizon plot representation66. b, High-resolution horizon graph of the phenotypic landscape of the HCMV genome. Shades of blue denote sensitization to host cell death, shades of red denote protection from cell death upon HCMV genome cleavage. Major features of the HCMV genome are annotated. sgRNAs targeting internal and terminal repeat regions (hashed) typically have multiple target sites and likely result in higher-order fragmentation of the HCMV genome, exacerbating their respective phenotypes. Viral ORFs are classified by their essentiality for viral replication based on ref. 10. ORFL150C, ORFL151C (originally named UL59, but thought to not be expressed as a protein67, causing it to be dropped from the consensus annotation), and ORFL152C were the only short ORFs with strong phenotypes in areas of the genome devoid of consensus genes. UL48 was the only gene that showed a substantial phenotype gradient within its gene body: Cutting the N-terminal region caused mild sensitization to death upon infection, whereas cutting the C-terminus had the opposite effect.
Extended Data Fig. 2 Host-directed CRISPRi and CRISPRn screen.
a, Results of the host-directed CRISPRn screen displayed as a scatter plot of average gene essentiality (that is infection-independent phenotype; y-axis) vs. protection/sensitization to death upon HCMV infection (that is infection-dependent phenotypes; x-axis), highlighting a manually selected set of hit genes. Note that due to the experimental design of the screen, the apparent gene essentiality phenotypes are underestimating the real essentiality because t0 refers to the beginning of HCMV infection, not lentiviral delivery of the sgRNA library. b, Direct comparison of CRISPRi and CRISPRn phenotypes, highlighting select host targets represented in both libraries. Hits involved in viral adhesion and entry are more pronounced in the CRISPRn screen. Cullin/RING pathway members and some vesicle trafficking factors were only resolved in the CRISPRi screen. See Supplemental Table 2 for a systematic comparison.
Extended Data Fig. 3 Single-cell infection time-course.
a, b, Numbers of cells, as well as distributions of UMIs per cell, percentage of viral transcripts per cell, and interferon score, broken down by cells for each MOI and each experimental time point (a), and for cells for each MOI and cluster membership (b). c, Distribution of cells across cell cycle phases in each of the clusters. d, Pearson’s correlation matrix of gene expression values (average logarithmized, scaled UMIs per gene per cell) for all clusters, broken down by low (L) and high (H) MOI conditions.
Extended Data Fig. 4 Viral gene expression profiles along the productive and abortive trajectory of infection.
a, Gene expression profiles for robustly detected viral genes along the dominant trajectory (clusters ‘infected 1–6’). Cells were grouped in bins spanning 2% of viral RNA and the gene expression (scaled UMIs per gene per cell) averaged for all cells in each bin. The heatmap shows the expression relative to the highest bin. Individual viral genes are ordered by similarity of the profiles, and annotated by genome position, phenotype of cutting within the body of a gene in the pooled virus-directed CRISPR screen (see Fig. 1 and Supplementary Fig. 1), and by the temporal profile as determined in a bulk proteomics study5. Note the relationship between a gene’s temporal class and its phenotype in the pooled screen: True-late and leaky-late genes predominantly showed protective phenotypes, whereas earlier classes also contained sensitizing genes. b, Gene expression profiles of viral genes along the abortive trajectory (clusters ‘infected 1–2’ and ‘infected abortive’). Cells were grouped in bins spanning 10% of viral RNA and the gene expression averaged for all cells in each bin. The heatmap shows the expression relative to the expression in an equivalent bin of the dominant trajectory.
Extended Data Fig. 5 Host-directed CRISPRi Perturb-seq experiment.
a, Numbers of single cells for each sgRNA target for each experimental time point in the host-directed CRISPRi Perturb-seq experiment. The average is 165 ± 50 (mean ± standard deviation) cells per sgRNA per time point. b, Knockdown levels for each sgRNA target calculated from the expression of the target gene in cells with a given sgRNA target relative to cells with control sgRNAs. No transcript at all was detected for VTCN1. Median knockdown level was 87.1%. c, Hierarchical clustering of expression changes of the most variable 100 genes (excluding the targeted factors) in response to host factor knockdown in naïve cells, relative to naïve cells with control sgRNAs. a–g, UMAP projections of single-cell transcriptomes of cells from the host-directed Perturb-seq experiment (same as in Fig. 4c), color-coded by experimental time post infection (d), percentage of viral transcripts per cell (e), interferon score, calculated from the normalized expression of interferon stimulated genes (f), and by pathway of the targeted host factor in each cell (g). h, Cluster membership as a function of sgRNA target and time post infection.
Extended Data Fig. 6 Host- and virus directed CRISPRn Perturb-seq experiment.
a, Numbers of single cells for each sgRNA target for each experimental time point in the host and virus-directed CRISPRn Perturb-seq experiment. The average is 188 ± 77 (mean ± standard deviation) cells per sgRNA per time point. Note the over-proportional drop in numbers in late time points of cells with apoptosis-related sgRNA targets. ‘Control’ denotes all safe-targeting sgRNAs, which are 4 and 5 distinct sgRNAs targeting the host and virus, respectively. b, Violin plots of the distribution of viral RNA fraction per cell as a function of time post infection and the sgRNA target (red, protective phenotype; blue, sensitizing phenotype; grey, control). Regions of the violin plot corresponding to uninfected cells, as well as early and late stages of infection are highlighted. Note that uninfected cells have non-zero background amounts of viral RNA, and those background levels are higher in later time points, indicating leaking of viral RNA from dying cells. c, d, Cluster membership as a function of sgRNA target and time post infection for cells with host-targeting sgRNAS (c) and virus-targeting sgRNAs (d). e–g, UMAP projections of single-cell transcriptomes of cells from the host and virus-directed Perturb-seq experiment (same as in Fig. 5c), color-coded by percentage of viral transcripts per cell (e), by pathway of the targeted host factor in each cell (f), and by viral target in each cell (g) for cells with host and viral targets, respectively.
Extended Data Fig. 7 Trajectories in viral gene expression space upon perturbation of viral factors.
a, Cartoon explaining the analytical workflow for comparing viral trajectories across the different sgRNA targets. b, Heatmaps of viral gene expression for all cells with virus-targeting sgRNAs, corresponding to the middle panel of the workflow cartoon. For each sgRNA target, cells were grouped in bins of 10% of viral RNA fraction, and the expression of viral genes plotted relative to a corresponding bin defined by cells with host-directed, safe-targeting sgRNAs (similar to Supplementary Fig. 4b), representing the unperturbed, dominant trajectory. Both the columns (viral sgRNA targets) as well as the rows (expressed viral genes) are ordered by genome position. This facilitates the distinction of gene expression effects in cis, that is the immediate effect of cutting on genes adjacent to the cut site, as opposed to in trans, which are reflecting an altered trajectory of infection. Pink boxes indicate the sgRNA target genes. c, Mapping the sgRNA targets onto the phenotypic landscape of the HCMV genome, indicating genome position and phenotype in the CRISPRn tiling screen.
Supplementary information
Supplementary Table 1
sgRNA sequences of the HCMV tiling library, raw sequencing counts in the screen and normalized guide-level phenotypes. Gene-level phenotypes for consensus genes (based on NCBI). Gene-level phenotypes for all ORFs, based on ref. 4.
Supplementary Table 2
Raw sequencing counts for the human genome-wide CRISPRi screens. Gene-level phenotypes for the human genome-wide CRISPRi screens, including significance scores calculated by MAGeCK58. Raw sequencing counts for the human genome-wide CRISPRn screen. Gene-level phenotypes for the human genome-wide CRISPRn screen, including significance scores calculated by MAGeCK. Comparison of gene-level phenotypes and MAGeCK scores between the CRISPRi and CRISPRn screen results.
Supplementary Table 3
Metadata annotations for all cells in the final dataset. Table of the expressed barcodes used to de-convolve the pooled cells into the experimental time points. Expression values of all robustly detected host and viral genes in the individual clusters. Expression values of robustly detected viral genes along the default trajectory of infection. Expression values of viral genes along the abortive trajectory of infection.
Supplementary Table 4
Metadata annotations for all cells in the final dataset. sgRNA sequences, guide barcodes and annotations for all elements of the library. Table of cell numbers in each cluster as a function of experimental time. Expression values of all robustly detected host genes in the naive cluster, as a function of sgRNA target. Expression values of all robustly detected host genes in the bystander cluster, as a function of sgRNA target.
Supplementary Table 5
Metadata annotations for all cells in the final dataset. sgRNA sequences, guide barcodes and annotations for all elements of the library. Table of cell numbers in each cluster as a function of experimental time. Expression values of all robustly detected viral genes along the trajectories of infection, as a function of sgRNA target.
Rights and permissions
About this article
Cite this article
Hein, M.Y., Weissman, J.S. Functional single-cell genomics of human cytomegalovirus infection. Nat Biotechnol 40, 391–401 (2022). https://doi.org/10.1038/s41587-021-01059-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41587-021-01059-3
This article is cited by
-
Essentiality and dynamic expression of the human tRNA pool during viral infection
Molecular Systems Biology (2026)
-
Molecular features of congenital cytomegalovirus infection in neonatal mouse brain at single-cell resolution
Acta Neuropathologica Communications (2025)
-
GraphVelo allows for accurate inference of multimodal velocities and molecular mechanisms for single cells
Nature Communications (2025)
-
Multidimensional analysis of host–virus interactions using the virus-encoded CRISPR-based direct readout system (VECOS)
Nature Protocols (2025)
-
Human cytomegalovirus exploits STING signaling and counteracts IFN/ISG induction to facilitate infection of dendritic cells
Nature Communications (2024)