Abstract
Optical pooled screening (OPS) is a scalable method for linking image-based phenotypes with cellular perturbations. However, it has thus far been restricted to relatively low-plex phenotypic readouts in cancer cell lines in culture due to limitations associated with in situ sequencing of perturbation barcodes. Here, we develop PerturbView, an OPS technology that leverages in vitro transcription to amplify barcodes before in situ sequencing, enabling screens with highly multiplexed phenotypic readouts across diverse systems, including primary cells and tissues. We demonstrate PerturbView in induced pluripotent stem cell-derived neurons, primary immune cells and tumor tissue sections from animal models. In a screen of immune signaling pathways in primary bone marrow-derived macrophages, PerturbView uncovered both known and novel regulators of NF-κB signaling. Furthermore, we combine PerturbView with spatial transcriptomics in tissue sections from a mouse xenograft model, paving the way to in situ screens with rich optical and transcriptomic phenotypes. PerturbView broadens the scope of OPS to a wide range of models and applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Screening and tissue images will be available on Image Data Resource (https://idr.openmicroscopy.org/). Additional data are available from the corresponding author upon request.
Code availability
Tissue analysis code is available at https://github.com/Genentech/PerturbView. Additional information required to reanalyze the data reported in this paper is available from the corresponding author on request.
Change history
26 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41587-025-02602-2
References
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR–Cas9 system. Science 343, 80–84 (2014).
Dixit, A. et al. Perturb-Seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Jaitin, D. A. et al. Dissecting immune circuits by linking CRISPR-pooled screens with single-cell RNA-seq. Cell 167, 1883–1896 (2016).
Adamson, B. et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167, 1867–1882 (2016).
Walton, R. T., Singh, A. & Blainey, P. C. Pooled genetic screens with image-based profiling. Mol. Syst. Biol. 18, e10768 (2022).
Wheeler, E. C. et al. Pooled CRISPR screens with imaging on microraft arrays reveals stress granule-regulatory factors. Nat. Methods 17, 636–642 (2020).
Lee, J. et al. Versatile phenotype-activated cell sorting. Sci. Adv. 6, eabb7438 (2020).
Yan, X. et al. High-content imaging-based pooled CRISPR screens in mammalian cells. J. Cell Biol. 220, e202008158 (2021).
Kanfer, G. et al. Image-based pooled whole-genome CRISPRi screening for subcellular phenotypes. J. Cell Biol. 220, e202006180 (2021).
Hasle, N. et al. High-throughput, microscope-based sorting to dissect cellular heterogeneity. Mol. Syst. Biol. 16, e9442 (2020).
Wang, C., Lu, T., Emanuel, G., Babcock, H. P. & Zhuang, X. Imaging-based pooled CRISPR screening reveals regulators of lncRNA localization. Proc. Natl Acad. Sci. USA 116, 10842–10851 (2019).
Feldman, D. et al. Optical pooled screens in human cells. Cell 179, 787–799 (2019).
Kudo, T., Lane, K. & Covert, M. W. A multiplexed epitope barcoding strategy that enables dynamic cellular phenotypic screens. Cell Syst. 13, 376–387 (2022).
Dhainaut, M. et al. Spatial CRISPR genomics identifies regulators of the tumor microenvironment. Cell 185, 1223–1239 (2022).
Carlson, R. J., Leiken, M. D., Guna, A., Hacohen, N. & Blainey, P. C. A genome-wide optical pooled screen reveals regulators of cellular antiviral responses. Proc. Natl Acad. Sci. USA 120, e2210623120 (2023).
Sansbury, S. E., Serebrenik, Y. V., Lapidot, T., Burslem, G. M. & Shalem, O. Pooled tagging and hydrophobic targeting of endogenous proteins for unbiased mapping of unfolded protein responses. Preprint at bioRxiv https://doi.org/10.1101/2023.07.13.548611 (2023).
Funk, L. et al. The phenotypic landscape of essential human genes. Cell 185, 4634–4653 (2022).
Ramezani, M. et al. A genome-wide atlas of human cell morphology. Preprint at bioRxiv https://doi.org/10.1101/2023.08.06.552164 (2023).
Sivanandan, S. et al. A pooled cell painting CRISPR screening platform enables de novo inference of gene function by self-supervised deep learning. Preprint at bioRxiv https://doi.org/10.1101/2023.08.13.553051 (2023).
Labitigan, R. L. D. et al. Mapping variation in the morphological landscape of human cells with optical pooled CRISPRi screening. eLife 13, RP94964 (2024).
Yamawaki, T. M. et al. Systematic comparison of high-throughput single-cell RNA-seq methods for immune cell profiling. BMC Genomics 22, 66 (2021).
Moffitt, J. R., Lundberg, E. & Heyn, H. The emerging landscape of spatial profiling technologies. Nat. Rev. Genet. 23, 741–759 (2022).
Hickey, J. W. et al. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. Nat. Methods 19, 284–295 (2022).
Askary, A. et al. In situ readout of DNA barcodes and single base edits facilitated by in vitro transcription. Nat. Biotechnol. 38, 66–75 (2020).
Choi, H. M. T. et al. Third-generation hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Radtke, A. J. et al. IBEX: an iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues. Nat. Protoc. 17, 378–401 (2022).
Datlinger, P. et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017).
Bernstein, B. E. et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 (2005).
Feldman, D. et al. Pooled genetic perturbation screens with image-based phenotypes. Nat. Protoc. 17, 476–512 (2022).
Conrad, T., Plumbom, I., Alcobendas, M., Vidal, R. & Sauer, S. Maximizing transcription of nucleic acids with efficient T7 promoters. Commun. Biol. 3, 439 (2020).
Romanienko, P. J. et al. A vector with a single promoter for in vitro transcription and mammalian cell expression of CRISPR gRNAs. PLoS ONE 11, e0148362 (2016).
Binan, L. et al. Simultaneous CRISPR screening and spatial transcriptomics reveals intracellular, intercellular, and functional transcriptional circuits. Preprint at bioRxiv https://doi.org/10.1101/2023.11.30.569494 (2023).
Preece, R. et al. ‘Mini’ U6 Pol III promoter exhibits nucleosome redundancy and supports multiplexed coupling of CRISPR/Cas9 effects. Gene Ther. 27, 451–458 (2020).
Yu, H., Lin, L., Zhang, Z., Zhang, H. & Hu, H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target. Ther. 5, 209 (2020).
Uemura, N. et al. TAK1 is a component of the Epstein–Barr virus LMP1 complex and is essential for activation of JNK but not of NF-κB. J. Biol. Chem. 281, 7863–7872 (2006).
Song, Z. et al. Roles of the kinase TAK1 in CD40-mediated effects on vascular oxidative stress and neointima formation after vascular injury. PLoS ONE 9, e101671 (2014).
Parnas, O. et al. A genome-wide CRISPR screen in primary immune cells to dissect regulatory networks. Cell 162, 675–686 (2015).
Black, S. et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat. Protoc. 16, 3802–3835 (2021).
Gut, G., Herrmann, M. D. & Pelkmans, L. Multiplexed protein maps link subcellular organization to cellular states. Science 361, eaar7042 (2018).
Lin, J.-R. et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife 7, e31657 (2018).
Eng, C.-H. L. et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature 568, 235–239 (2019).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Cleary, B. et al. Compressed sensing for highly efficient imaging transcriptomics. Nat. Biotechnol. 39, 936–942 (2021).
Lin, J.-R., Fallahi-Sichani, M. & Sorger, P. K. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat. Commun. 6, 8390 (2015).
Park, J. et al. Spatial omics technologies at multimodal and single cell/subcellular level. Genome Biol. 23, 256 (2022).
Moon, K. R. et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 37, 1482–1492 (2019).
Gao, M. H. et al. Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J. Biol. Chem. 279, 38797–38802 (2004).
Castanza, A. S. et al. Extending support for mouse data in the Molecular Signatures Database (MSigDB). Nat. Methods 20, 1619–1620 (2023).
Corcoran, S. E. & O’Neill, L. A. J. HIF1α and metabolic reprogramming in inflammation. J. Clin. Invest. 126, 3699–3707 (2016).
Yao, D. et al. Scalable genetic screening for regulatory circuits using compressed Perturb-Seq. Nat. Biotechnol. 42, 1282–1295 (2023).
Seferbekova, Z., Lomakin, A., Yates, L. R. & Gerstung, M. Spatial biology of cancer evolution. Nat. Rev. Genet. 24, 295–313 (2023).
Wagner, D. E. & Klein, A. M. Lineage tracing meets single-cell omics: opportunities and challenges. Nat. Rev. Genet. 21, 410–427 (2020).
Lamprecht, S. et al. Multicolor lineage tracing reveals clonal architecture and dynamics in colon cancer. Nat. Commun. 8, 1406 (2017).
Janesick, A. et al. High resolution mapping of the breast cancer tumor microenvironment using integrated single cell, spatial and in situ analysis of FFPE tissue. Nat. Commun. 14, 8353 (2023).
Lorenzini, A. et al. Significant correlation of species longevity with DNA double strand break recognition but not with telomere length. Mech. Ageing Dev. 130, 784–792 (2009).
UC San Diego Broad Institute. Human gene set: hallmark mitotic spindle. Gene Set Enrichment Analysis https://www.gsea-msigdb.org/gsea/msigdb/human/geneset/HALLMARK_MITOTIC_SPINDLE.html (2015).
Way, G. P. et al. Morphology and gene expression profiling provide complementary information for mapping cell state. Cell Syst. 13, 911–923 (2022).
Cheng, Y. et al. Perturb-tracing enables high-content screening of multiscale 3D genome regulators. Preprint at bioRxiv https://doi.org/10.1101/2023.01.31.525983 (2023).
Soares, R. R. G. et al. Pooled optical screening in bacteria using chromosomally expressed barcodes. Preprint at bioRxiv https://doi.org/10.1101/2023.11.17.567382 (2023).
Adamson, B., Norman, T. M., Jost, M. & Weissman, J. S. Approaches to maximize sgRNA-barcode coupling in Perturb-Seq screens. Preprint at bioRxiv https://doi.org/10.1101/298349 (2018).
Feldman, D., Singh, A., Garrity, A. J. & Blainey, P. C. Lentiviral co-packaging mitigates the effects of intermolecular recombination and multiple integrations in pooled genetic screens. Preprint at bioRxiv https://doi.org/10.1101/262121 (2018).
Zheng, X. et al. Massively parallel in vivo Perturb-Seq reveals cell-type-specific transcriptional networks in cortical development. Cell 187, 3236–3248 (2024).
Jin, X. et al. In vivo Perturb-Seq reveals neuronal and glial abnormalities associated with autism risk genes. Science 370, eaaz6063 (2020).
Liu, B. et al. Large-scale multiplexed mosaic CRISPR perturbation in the whole organism. Cell 185, 3008–3024 (2022).
Molbay, M., Kolabas, Z. I., Todorov, M. I., Ohn, T.-L. & Ertürk, A. A guidebook for DISCO tissue clearing. Mol. Syst. Biol. 17, e9807 (2021).
Hontani, Y., Xia, F. & Xu, C. Multicolor three-photon fluorescence imaging with single-wavelength excitation deep in mouse brain. Sci. Adv. 7, eabf3531 (2021).
Nehme, R. et al. Combining NGN2 programming with developmental patterning generates human excitatory neurons with NMDAR-mediated synaptic transmission. Cell Rep. 23, 2509–2523 (2018).
Chailangkarn, T. et al. A human neurodevelopmental model for Williams syndrome. Nature 536, 338–343 (2016).
Wang, G. G. et al. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293 (2006).
Luchetti, G. et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe 29, 1521–1530 (2021).
Chen, H. et al. Population-wide gene disruption in the murine lung epithelium via AAV-mediated delivery of CRISPR–Cas9 components. Mol. Ther. Methods Clin. Dev. 27, 431–449 (2022).
Platt, R. J. et al. CRISPR–Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Deich, C. et al. T7Max transcription system. J. Biol. Eng. 17, 4 (2023).
Hoberecht, L., Perampalam, P., Lun, A. & Fortin, J.-P. A comprehensive Bioconductor ecosystem for the design of CRISPR guide RNAs across nucleases and technologies. Nat. Commun. 13, 6568 (2022).
Schwartz, J. J., Lee, C. & Shendure, J. Accurate gene synthesis with tag-directed retrieval of sequence-verified DNA molecules. Nat. Methods 9, 913–915 (2012).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://doi.org/10.48550/ARXIV.1303.3997 (2013).
Peng, T. et al. A BaSiC tool for background and shading correction of optical microscopy images. Nat. Commun. 8, 14836 (2017).
Muhlich, J. L. et al. Stitching and registering highly multiplexed whole-slide images of tissues and tumors using ASHLAR. Bioinformatics 38, 4613–4621 (2022).
Vaskivskyi, V. Microaligner: image registration (alignment) software for large microscopy images. GitHub https://github.com/VasylVaskivskyi/microaligner (2022).
Schmidt, U., Weigert, M., Broaddus, C. & Myers, G. Cell detection with star-convex polygons. In Medical Image Computing and Computer Assisted Intervention, MICCAI 2018 (eds. Frangi, A. F., Schnabel, J. A., Davatzikos, C., Alberola-Lopez, C. & Fichtinger, G.) 265–273 (Springer International Publishing, 2018).
Weigert, M., Schmidt, U., Haase, R., Sugawara, K. & Myers, G. Star-convex polyhedra for 3D object detection and segmentation in microscopy. In 2020 IEEE Winter Conference on Applications of Computer Vision (WACV) 3655–3662 (IEEE, 2020).
Su, Z. et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J. Mol. Diagn. 13, 74–84 (2011).
Jalili, V., Matteucci, M., Masseroli, M. & Morelli, M. J. Using combined evidence from replicates to evaluate ChIP–seq peaks. Bioinformatics 31, 2761–2769 (2015).
van der Walt, S. et al. scikit-image: image processing in Python. PeerJ 2, e453 (2014).
Stoeckius, M. et al. Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19, 224 (2018).
Ando, D. M., McLean, C. Y. & Berndl, M. Improving phenotypic measurements in high-content imaging screens. Preprint at bioRxiv https://doi.org/10.1101/161422 (2017).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Celik, S. et al. Biological cartography: building and benchmarking representations of life. Preprint at bioRxiv https://doi.org/10.1101/2022.12.09.519400 (2022).
Bray, M.-A., Fraser, A. N., Hasaka, T. P. & Carpenter, A. E. Workflow and metrics for image quality control in large-scale high-content screens. J. Biomol. Screen. 17, 266–274 (2012).
Patterson, H. & Manz, T. wsireg: multimodal whole slide image registration in a graph structure. GitHub https://github.com/NHPatterson/wsireg (2022).
Allan, D. B., Caswell, T., Keim, N. C., van der Wel, C. M. & Verweij, R. W. Soft-Matter/Trackpy: V0.6.1. Zenodo https://doi.org/10.5281/ZENODO.7670439 (2023).
Fukai, Y. T. M2Stitch. Zenodo https://doi.org/10.5281/ZENODO.5139596 (2021).
Acknowledgements
We thank L. Gaffney and A. Hupalowska for their help in figure making. Schematic figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
T.K., E.L. and A.R. conceived the study. T.K. and E.L. designed the study, experiments and analysis and interpreted data. T.K., R.M. and Y.C. performed ISS in tumors. A.M.M. and P.C. advised on and supported macrophage screens. P.C., V.C. and N. Kayagaki advised on the interpretation of screens. M.H., N. Kljavin, J.J., F.d.S.M. and L.M. advised on and performed animal experiments. P.W. performed MCF7 experiments. P.W., A.S.C. and A.S. supported microscopy. X.H., O.K., N. Kayagaki, V.C., C.C., M.M., P.C., P.T. and K.G.-S. advised on and provided cell lines. C.S.L. and S.C. supported optimization of multiplexed imaging. T.K., R.J., C.F., H.C.B., J.P.T., R.W. and E.L. analyzed tissue data. T.K., B.H. and A.R. performed multimodal screen analysis. J.G., J.S.H., B.L., E.B.-G., D.R. and E.L. advised on and supported analysis. T.K., E.L., A.S. and A.R. wrote the paper, and E.L., L.G., O.R.-R. and A.R. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
Genentech has submitted a provisional patent application that is based on the technology described in this manuscript. All authors are or were employed by Genentech, Inc., South San Francisco, California, at the time of their contribution to this work. A.R. is a cofounder and equity holder of Celsius Therapeutics and an equity holder in Immunitas and, until 31 July 2020, was a scientific advisory board member of Thermo Fisher Scientific, Syros Pharmaceuticals, Neogene Therapeutics and Asimov. T.K. is a shareholder of Genomelink, Inc. E.L. is an equity holder in insitro, Inc. A.M.M., R.M., Y.C., P.W., J.G., X.H., O.K., R.J., C.F., B.H., H.C.B., J.P.T., R.W., A.S.C., M.M., C.C., N. Kljavin, J.J., D.R., P.T., E.B.-G., K.G.-S., J.S.H., N. Kayagaki, F.d.S.M., L.M., B.L., A.S., L.G., O.R.-R., A.R. and E.L. are equity holders in Roche.
Peer review
Peer review information
Nature Biotechnology thanks Howard Chang, Prisca Liberali and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Establishment of PerturbView.
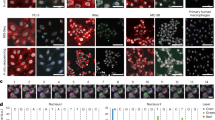
a, Varied activity of CROP-Seq vector by HCR FISH in primary cells. Left, HCR FISH of CROP-Seq expressed transcript (green; detected with FISH probes against puromycin and U6 promoter regions; Supplementary Table 7) across different mouse cell lines (scale bar, 200 μm). Right, CROP-Seq transcript density in A549 (left) and mouse BMDM (right) cells (scale bar, 50 μm). All cells were transduced at low MOI and puromycin-selected. b, T7 promoter position dramatically affects IVT-enhanced ISS signal. Distribution of median single cell nuclear intensity following IVT in the original U6 promoter (top; gray, n = 62,321 cells pooled from three well replicates), T7 promoter in place of the U6 promoter (orange, n = 48,123), and T7 promoter upstream of U6 promoter (blue, n = 53,777). Bottom, representative cellular images from each construct (scale bar, 25 μm). c, Example gating strategy for CD81 flow cytometry assays. d, The U6/T7 promoter32 has lower CRISPR editing activity than the unmodified U6 promoter. The distribution of CD81 expression by flow cytometry for each construct. The fraction of knocked-out cells (thresholded by the vertical line) is noted on the left. k = 34,950 cells, at least 11,247 cells per condition. e, Editing efficiency of the PerturbView vector is identical to wild-type U6 promoter in A549 cells, immortalized macrophages and fibroblasts. Distribution of target gene expression of each construct in A549 cells (top; CD81, n = 3 independent infection replicates combined; 876,505 cells, at least 61,250 cells per replicate and condition), primary IMR90 fibroblasts (bottom; CD81, 17,497 cells, at least 5,524 cells per condition) and immortalized macrophages (right; CD44, sg1 and sg2: separate guide replicates; 126,093 cells, at least 20,844 cells per condition). The fraction of knocked-out cells (thresholded by the vertical line) is noted on the left. f, g, Decrosslinking and IVT duration impact sensitivity and precision of barcode detection. Bottom, mean sensitivity (purple) and precision (blue)at different durations of decrosslinking (f, n = 4 well replicates (dots), at least 9,393 cells per replicate) or IVT (g, n = 4 well replicates (dots), at least 9,387 cells per replicate). Error bars: SD. Top, representative cellular images from each condition (scale bar, 25 μm).
Extended Data Fig. 2 Optimization of PerturbView.
a, PerturbView with methanol fixation. Mean sensitivity (blue) and precision (orange) with (65 °C) or without (25 °C) decrosslinking in methanol-fixed cells (left) or PFA-fixed cells (right) (n = 2 independent replicates). b, PerturbView enables in situ sequencing at 4x magnification. Left, Whole well (top; scale bar, 1 mm) or zoomed image (bottom; scale bar, 50 μm) representative image acquired at 4X magnification with either conventional ISS (left) or PerturbView (right). All images are contrasted identically. Heat decrosslinking prior to PerturbView results in increased nuclear DAPI intensity. c,d, DTT rescues IVT efficiency at low T7 polymerase concentration in MCF7 cells. c, representative cell images from each condition (scale bar, 25 μm). d, Mean maximum normalized intensity of in situ sequencing spots (y axis) at different T7 polymerase concentrations (x axis) with (orange, n = 3 technical replicates) or without (grey, n = 2 technical replicates) DTT.
Extended Data Fig. 3 Context and phenotype specific perturbation effects in PerturbView screen in primary immune cells.
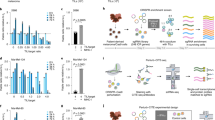
a, p65 staining based assay. Representative images cells stained with anti-p65 antibody (top) and their segmented nuclei and cytoplasm (bottom, green and magenta, respectively). Scale bar, 25 µm. b, Agreement in sgRNA detection in PerturbView and NGS. PerturbView ISS cell counts (y axis) and NGS read counts of the cell library (x axis) for each perturbation barcode (dot). Top left: Pearson’s r. c–f, Shared and stimulation-specific perturbation effects. c, Intersection of hit genes between TNFα, IL1β, and LPS PerturbView screens (circles) of NFΚB translocation in primary BMDMs. d–f, Cumulative distribution functions (CDFs) of robust z-scores for p65 nuclear intensity in response to LPS (left), TNFα (middle), or IL1β (right) for each of four guides (colored curves; n = cell number per guide) targeting the genes Map3k7 (d), Prkar1a (e), and Tnfrsf1a (f), compared to cells with non-targeting or non-essential controls (Methods; gray line (combined); shading standard deviation of robust z-scores).
Extended Data Fig. 4 Multimodal PerturbView screen in primary immune cells.
a, PerturbView enables efficient sgRNA recovery after different multiplexed imaging techniques. Left, BMDMs were stained with F4/80 repeatedly using 4i, IBEX and cycIF (Scale Bar, 50 μm). Middle, Representative images of in situ sequencing results after 6 rounds of staining (Scale Bar, 50 μm). Right, Mean percentage of cells positive for sgRNA signal (y axis, ≥1 sgRNA read per cell, mean of well replicates) after each staining step (x axis). Error bar, SD. n = 2 independent replicates. b, Agreement in phenotypic profiles of different guides targeting the same gene. PHATE embeddings of RNA/protein joint profiles (dots) for each sgRNAs in the TNFα (left) or IL1β (right) screen targeting a gene (color) or controls (grey). Gene names are shown for each guide whose 5 nearest neighbors contain another guide targeting the same gene. c, Distribution of cosine similarity of the phenotypic profiles derived from joint RNA/protein profiling for random pairs of guides (“random”, blue) and either guides targeting the same gene across all genes (orange; “same gene”, top) or targeting Olfr genes (orange “same gene”, bottom). d, e, Contribution of different molecular phenotypes to perturbation impact. d, Impact score (FDR based on one-sided tests adjusted with Benjamini-Hochberg method, color bar) for each perturbed gene (row) in the TNFα (top) or IL1β (bottom) screen, when assessed only based on one imaging feature (single-channel, left) or all other features (dropping a channel, right). Rows and columns are clustered by hierarchical clustering. e, Top, CDFs of phospho-rpS6 intensity (x axis) in response to TNFα for four guides (colored curves), compared to cells with non-targeting or non-essential controls (gray line (mean); shading standard deviation). FDRs were computed for the genes listed (in the rows) in d. Bottom, representative phospho-rpS6 images under perturbations. Two cells were sampled from each of a 10-percentile group (according to the mean cellular phospho-rpS6 level) and arranged from low (left) to high (right).
Extended Data Fig. 5 PerturbView in tissue.
a–d, High barcoding decoding accuracy in tissue sections with PerturbView. a, Barcode mapping rate (y axis, fraction of assigned reads at a Hamming distance ≤1 to the pre-defined sgRNA lookup table) for conventional ISS and PerturbView (x axis) in FF and FFPE tumor tissue (n = 4). b, Mean percentage of cells with detectable sgRNA (y axis) captured by conventional ISS or by PerturbView (x axis) in FFPE (light green) or fresh frozen (FF) (dark green) subcutaneous tumors. n = 2 independent experiments, Error bars: SD. c, Representative image of immunofluorescence stain with anti-human PRKDC in DLD-1 xenograft tissue section. Mouse cells (outer rim) or necrotic regions (central region) show low PRKDC staining. Scale bar, 1 mm. d, Frequency of each sgRNA (dots; normalized counts) estimated by NGS of the plasmid library (x axis) or PerturbView (y axis) of FF (dark green) or FFPE (light green) samples. n = 2 for each FF and FFPE treatment. Pearson’s r is noted. e, Spatial transcriptome and barcode detection in UMAP and spatial domains. UMAP embedding on transcriptome profiles (dots) colored and numbered by Leiden clusters (far left); and tissue section colored by the same Leiden clusters (second from left), total transcript counts in each cell (second from right) and minimum barcode hamming distance (up to 2, right); and distribution of total transcript counts per cell (x axis) for each Leiden cluster (y axis). Interior box plots indicate the median (white lines), interquartile range (hinges) and 1.5 times of the interquartile range (whiskers). (n = 2 consecutive sections per tumor). f, g, Expression changes associated with clonal diversity. f, Tumor sections profiled (scale bar, 1 mm), colored by Shannon diversity (top) or corresponding diversity groups (bottom) for all analyzed sections aside from the one shown in Fig. 3g. g, Genes (columns) that are differentially expressed (log fold-change > 0.5) between regions with high and low Shannon diversity (rows) for each tumor section (n = 2 consecutive sections per tumor).
Supplementary information
Supplementary Information
PerturbView protocols.
Supplementary Tables 1–7
Supplementary Table 1. Sequences of U6 promoter variants. Supplementary Table 2. sgRNA library information. Supplementary Table 3. Summary of p65 regulator screens. For each stimulation, perturbed gene (gene_name), cell counts (count), median p65 intensity (nuclei_median), P values computed with the two-sided empirical CDF method (pval) and FDR (pvals_corrected) are listed. Supplementary Table 4. Summary of joint RNA and protein screens. For each gene perturbation (gene_symbol), a type of stimulation (stimulation), Leiden cluster number (cluster), P values (pval), FDR (pval_bh) and test results (Is_significant) are listed. Supplementary Table 5. PHATE map embedding locations of each gene perturbations. Supplementary Table 6. Primers and sgRNA sequences. Supplementary Table 7. HCR FISH probe sequences.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kudo, T., Meireles, A.M., Moncada, R. et al. Multiplexed, image-based pooled screens in primary cells and tissues with PerturbView. Nat Biotechnol 43, 1091–1100 (2025). https://doi.org/10.1038/s41587-024-02391-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41587-024-02391-0
This article is cited by
-
Massively parallel in vivo Perturb-seq screening
Nature Protocols (2025)
-
Spatial profiling of gene editing by in situ sequencing in mice and macaques
Nature Biomedical Engineering (2025)
-
Integrated in vivo combinatorial functional genomics and spatial transcriptomics of tumours to decode genotype-to-phenotype relationships
Nature Biomedical Engineering (2025)
-
Engineering next-generation microfluidic technologies for single-cell phenomics
Nature Genetics (2025)
-
Transcriptome-wide analysis of differential expression in perturbation atlases
Nature Genetics (2025)