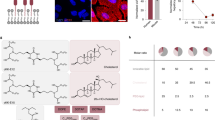
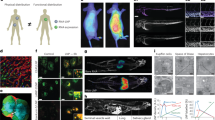
Abstract
Transplantation of ex vivo engineered hematopoietic stem cells (HSCs) can lead to robust clinical responses but carries risks of adverse events from bone marrow mobilization, chemotherapy conditioning and other factors. HSCs have been modified in vivo using lipid nanoparticles (LNPs) decorated with targeting moieties, which increases manufacturing complexity. Here we screen 105 LNPs without targeting ligands for effective homing to the bone marrow in mouse. We report an LNP named LNP67 that delivers mRNA to murine HSCs in vivo, primary human HSCs ex vivo and CD34+ cells in rhesus monkeys (Macaca mulatta) in vivo at doses of 0.25 and 0.4 mg kg−1. Without mobilization and conditioning, LNP67 can mediate delivery of mRNA to HSCs and their progenitor cells (HSPCs), as well as to the liver in rhesus monkeys, without serum cytokine activation. These data support the hypothesis that in vivo delivery to HSCs and HSPCs in nonhuman primates is feasible without targeting ligands.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
scRNA-seq raw data were deposited to the National Center for Biotechnology Information Sequence Read Archive database (SRR28416440, SRR28416441, SRR28416442, SRR28417638 and SRR28417639) under project identifier PRJNA1090779. The mouse genome GRCm39 can be accessed at https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000001635.27/. All other data are shown.
References
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2020).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
Verve Therapeutics doses first human with an investigational in vivo base editing medicine, VERVE-101, as a potential treatment for heterozygous familial hypercholesterolemia. Verve Therapeutics https://ir.vervetx.com/news-releases/news-release-details/verve-therapeutics-doses-first-human-investigational-vivo-base (2022).
Longhurst, H. J. et al. CRISPR–Cas9 in vivo gene editing of KLKB1 for hereditary angioedema. N. Engl. J. Med. 390, 432–441 (2024).
Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216–234 (2021).
Salinas Cisneros, G. & Thein, S. L. Recent advances in the treatment of sickle cell disease. Front. Physiol. 11, 435 (2020).
Taher, A. T., Musallam, K. M. & Cappellini, M. D. β-thalassemias. N. Engl. J. Med. 384, 727–743 (2021).
Cazzola, M. Introduction to a review series on inherited anemias. Blood 136, 1215–1216 (2020).
Castagnoli, R., Delmonte, O. M., Calzoni, E. & Notarangelo, L. D. Hematopoietic stem cell transplantation in primary immunodeficiency diseases: current status and future perspectives. Front. Pediatr. 7, 295 (2019).
Tan, E. Y., Boelens, J. J., Jones, S. A. & Wynn, R. F. Hematopoietic stem cell transplantation in inborn errors of metabolism. Front. Pediatr. 7, 433 (2019).
Eichler, F. et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 377, 1630–1638 (2017).
Frangoul, H. et al. CRISPR–Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
Locatelli, F. et al. Betibeglogene autotemcel gene therapy for non-β0/β0 genotype β-thalassemia. N. Engl. J. Med. 386, 415–427 (2021).
Kanter, J. et al. Biologic and clinical efficacy of LentiGlobin for sickle cell disease. N. Engl. J. Med. 386, 617–628 (2022).
Domingues, M. J., Nilsson, S. K. & Cao, B. New agents in HSC mobilization. Int. J. Hematol. 105, 141–152 (2017).
Atilla, E., Ataca Atilla, P. & Demirer, T. A review of myeloablative vs reduced intensity/non-myeloablative regimens in allogeneic hematopoietic stem cell transplantations. Balkan Med. J. 34, 1–9 (2017).
Shi, D., Toyonaga, S. & Anderson, D. G. In vivo RNA delivery to hematopoietic stem and progenitor cells via targeted lipid nanoparticles. Nano Lett. 23, 2938–2944 (2023).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023).
Breda, L. et al. In vivo modification of hematopoietic stem cells by targeted lipid nanoparticles encapsulating mRNA. Blood 140, 305–306 (2022).
Li, C. et al. Safe and efficient in vivo hematopoietic stem cell transduction in nonhuman primates using HDAd5/35++ vectors. Mol. Ther. Methods Clin. Dev. 24, 127–141 (2022).
Mui, B. L. et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2, e139 (2013).
Eygeris, Y., Patel, S., Jozic, A. & Sahay, G. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 20, 4543–4549 (2020).
Kulkarni, J. A., Witzigmann, D., Leung, J., Tam, Y. Y. C. & Cullis, P. R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 11, 21733–21739 (2019).
Kauffman, K. J. et al. Rapid, single-cell analysis and discovery of vectored mRNA transfection in vivo with a loxP-flanked tdTomato reporter mouse. Mol. Ther. Nucleic Acids 10, 55–63 (2018).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
LoPresti, S. T., Arral, M. L., Chaudhary, N. & Whitehead, K. A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilities targeted mRNA delivery to the spleen and lungs. J. Control. Release 345, 819–831 (2022).
Radmand, A. et al. The transcriptional response to lung-targeting lipid nanoparticles in vivo. Nano Lett. 23, 993–1002 (2023).
Paunovska, K. et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 18, 2148–2157 (2018).
Dobrowolski, C. et al. Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery. Nat. Nanotechnol. 17, 871–879 (2022).
Tiwari, P. M. et al. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat. Commun. 9, 3999 (2018).
Hatit, M. Z. C. et al. Species-dependent in vivo mRNA delivery and cellular responses to nanoparticles. Nat. Nanotechnol. 17, 310–318 (2022).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Jurecic, R. Hematopoietic stem cell heterogeneity. Adv. Exp. Med. Biol. 1169, 195–211 (2019).
Kim, J., Eygeris, Y., Ryals, R. C., Jozić, A. & Sahay, G. Strategies for non-viral vectors targeting organs beyond the liver. Nat. Nanotechnol. 19, 428–447 (2023).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Le, T. et al. BBrowser: making single-cell data easily accessible. Preprint at bioRxiv https://doi.org/10.1101/2020.12.11.414136 (2020).
Moreno, A. et al. Anti-PEG antibodies inhibit the anticoagulant activity of PEGylated aptamers. Cell Chem. Biol. 26, 634–644 (2019).
Urits, I. et al. A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol. Ther. 9, 301–315 (2020).
FDA cellular & gene therapy guidances. US Food and Drug Administration https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances (2022).
Tarantal, A. F., Noctor, S. C. & Hartigan-O’Connor, D. J. Nonhuman primates in translational research. Annu. Rev. Anim. Biosci. 10, 441–468 (2022).
Lindsay, K. E. et al. Aerosol delivery of synthetic mRNA to vaginal mucosa leads to durable expression of broadly neutralizing antibodies against HIV. Mol. Ther. 28, 805–819 (2020).
Sago, C. D. et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl Acad. Sci. USA 115, E9944–E9952 (2018).
Chen, D. et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 134, 6948–6951 (2012).
Belliveau, N. M. et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids 1, e37 (2012).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Dahlman, J. E. et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 9, 648–655 (2014).
Sager, H. B. et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 8, 342ra80 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337(2019).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Ni, H. et al. Piperazine-derived lipid nanoparticles deliver mRNA to immune cells in vivo. Nat. Commun. 13, 4766 (2022).
Tarantal, A. F. in Handbook of Experimental Animals, The Laboratory Primate (ed. Wolfe-Coote, S.) 317–352 (Elsevier Academic Press, 2005).
Tarantal, A. F. et al. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol. Ther. 20, 1033–1045 (2012).
Guo, C., Liu, W., Hua, X., Li, H. & Jia, S. Fourier light-field microscopy. Opt. Express 27, 25573–25594 (2019).
Hua, X., Liu, W. & Jia, S. High-resolution Fourier light-field microscopy for volumetric multi-color live-cell imaging. Optica 8, 614–620 (2021).
Sternberg, S. Biomedical image processing. Computer 16, 22–34 (1983).
Mandracchia, B. et al. Fast and accurate sCMOS noise correction for fluorescence microscopy. Nat. Commun. 11, 94 (2020).
Saha, K. et al. The NIH somatic cell genome editing program. Nature 592, 195–204 (2021).
Acknowledgements
We thank K. Tiegren at Emory University for copyediting the manuscript. We thank R. C. Guerrero-Ferreira at the Emory University Robert P. Apkarian Integrated Electron Microscopy Core Facility (RRID: SCR_023537) for cryo-TEM imaging. The cryo-TEM data described here were collected on the JEOL JEM-2200FS 200-kV TEM instrument supported by the National Science Foundation Major Research Instrumentation (grant 0923395). We thank P. Bagchi at the Emory Integrated Proteomics Core (EIPC) subsidized by the Emory University School of Medicine for the proteomics assay. For the proteomics assay at EIPC, additional support was provided by the Georgia Clinical and Translational Science Alliance of the National Institutes of Health (NIH; award number UL1TR002378). These studies were supported through the NIH Somatic Cell Genome Editing (SCGE) consortium. This was a collaborative effort between UH3-TR002855 (J.E.D. and P.J.S.) and the Nonhuman Primate Testing Center for Evaluation of Somatic Cell Genome-Editing Tools (U42 OD027094, to A.T.). Information about these studies is also provided in the SCGE toolkit, supported through the SCGE Dissemination and Coordinating Center (https://commonfund.nih.gov/editing)63. Studies were also supported through the base operating grant for the California National Primate Research Center (P51-OD011107). This work was funded in part through ATLANTIS NIH training grant in kidney, urology and hematology (TL1DK136047, to R.Z.).
Author information
Authors and Affiliations
Contributions
H.K., R.Z., A.T. and J.E.D. designed the experiments and analyzed data. H.K. and R.Z. led all experiments. A.T. and J.E.D. supervised the project. K.G., L.L., S.G.H., A.R., A.L. and A.S. assisted with mouse experiments. K.G. and L.L. assisted with LNP characterization. D.L. performed next-generation sequencing. A.R.P., M.Z.C.H. and H.N. designed and synthesized the ionizable lipid. H.E.P. and P.J.S. designed and synthesized the mRNA. K.H., X.H. and S.J. performed Fourier light-field fluorescence microscopy imaging of rhesus cells. M.M., C.L. and A.T. performed rhesus macaque experiments. H.K., R.Z., A.F.T. and J.E.D. wrote the initial draft, which was sent to all authors.
Corresponding author
Ethics declarations
Competing interests
M.Z.C.H., H.N. and J.E.D. have filed a provisional patent related to this manuscript (US patent application number 63/632,354). J.E.D. is an advisor to GV, Readout, Edge Animal Health and Nava Therapeutics and P.J.S. is a cofounder of Tether Therapeutics. None of these companies provided any financial support for this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19 and references.
Supplementary Table 1
Sequences for LNP barcodes and templates for mRNA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Zenhausern, R., Gentry, K. et al. Lipid nanoparticle-mediated mRNA delivery to CD34+ cells in rhesus monkeys. Nat Biotechnol 43, 1813–1820 (2025). https://doi.org/10.1038/s41587-024-02470-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41587-024-02470-2
This article is cited by
-
Targeted delivery of genome editors in vivo
Nature Biotechnology (2026)
-
Design principles of lipid nanoparticles for RNA delivery
Nature Reviews Bioengineering (2026)
-
Lipid nanoparticle screening in nonhuman primates with minimal loss of life
Nature Biotechnology (2025)
-
Dual switches ignite tumour-specific mRNA therapeutics
Nature Nanotechnology (2025)
-
Next-generation materials for nucleic acid delivery
Nature Reviews Materials (2025)