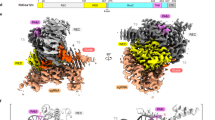
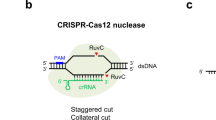
Abstract
CRISPR-associated Cas12 proteins are a highly variable collection of nucleic acid-targeting proteins. All Cas12 variants use RNA guides and a single nuclease domain to target complementary DNA or, in rare cases, RNA. The high variability of Cas12 effectors can be explained by a series of independent evolution events from different transposon-associated TnpB-like ancestors. Despite basic structural and functional similarities, this has resulted in unprecedented variation of the Cas12 effector proteins in terms of size, domain composition, guide structure, target identity and interference strategy. In this Review, we compare the unique molecular features of natural and engineered Cas12 and TnpB variants. Furthermore, we provide an overview of established genome editing and diagnostic applications and discuss potential future directions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
van der Oost, J. in CRISPR: Biology and Applications (eds Barrangou, R., Sontheimer, E. J. & Marraffini, L. A.) Ch. 3 (Wiley, 2022).
van der Oost, J., Jore, M. M., Westra, E. R., Lundgren, M. & Brouns, S. J. CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem. Sci. 34, 401–407 (2009).
Mohanraju, P. et al. Diverse evolutionary roots and mechanistic variations of the CRISPR–Cas systems. Science 353, aad5147 (2016).
Jinek, M. et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR–Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell 163, 759–771 (2015).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400 (2008).
Garneau, J. E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Deltcheva, E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Gasiunas, G., Barrangou, R., Horvath, P. & Siksnys, V. Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl Acad. Sci. USA 109, E2579–E2586 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cho, S. W., Kim, S., Kim, J. M. & Kim, J. S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Gasiunas, G. et al. A catalogue of biochemically diverse CRISPR–Cas9 orthologs. Nat. Commun. 11, 5512 (2020).
Aliaga Goltsman, D. S. et al. Compact Cas9d and HEARO enzymes for genome editing discovered from uncultivated microbes. Nat. Commun. 13, 7602 (2022).
Altae-Tran, H. et al. The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases. Science 374, 57–65 (2021).
Makarova, K. S. & Koonin, E. V. Annotation and classification of CRISPR–Cas systems. Methods Mol. Biol. 1311, 47–75 (2015).
Teng, F. et al. Repurposing CRISPR–Cas12b for mammalian genome engineering. Cell Discov. 4, 63 (2018).
Wu, W. Y. et al. The miniature CRISPR–Cas12m effector binds DNA to block transcription. Mol. Cell 82, 4487–4502 (2022).
Koonin, E. V., Makarova, K. S. & Zhang, F. Diversity, classification and evolution of CRISPR–Cas systems. Curr. Opin. Microbiol. 37, 67–78 (2017).
Altae-Tran, H. et al. Diversity, evolution, and classification of the RNA-guided nucleases TnpB and Cas12. Proc. Natl Acad. Sci. USA 120, e2308224120 (2023).
Capdeville, N., Schindele, P. & Puchta, H. Getting better all the time—recent progress in the development of CRISPR/Cas-based tools for plant genome engineering. Curr. Opin. Biotechnol. 79, 102854 (2023).
Huang, C. H., Lee, K. C. & Doudna, J. A. Applications of CRISPR–Cas enzymes in cancer therapeutics and detection. Trends Cancer 4, 499–512 (2018).
Donohoue, P. D., Barrangou, R. & May, A. P. Advances in industrial biotechnology using CRISPR–Cas systems. Trends Biotechnol. 36, 134–146 (2018).
Kersulyte, D., Mukhopadhyay, A. K., Shirai, M., Nakazawa, T. & Berg, D. E. Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. J. Bacteriol. 182, 5300–5308 (2000).
Karvelis, T. et al. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature 599, 692–696 (2021).
Ton-Hoang, B. et al. Single-stranded DNA transposition is coupled to host replication. Cell 142, 398–408 (2010).
Meers, C. et al. Transposon-encoded nucleases use guide RNAs to promote their selfish spread. Nature 622, 863–871 (2023).
Saito, M. et al. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 620, 660–668 (2023).
Jiang, K. et al. Programmable RNA-guided DNA endonucleases are widespread in eukaryotes and their viruses. Sci. Adv. 9, eadk0171 (2023).
Aravind, L., Makarova, K. S. & Koonin, E. V. Holliday junction resolvases and related nucleases: identification of new families, phyletic distribution and evolutionary trajectories. Nucleic Acids Res. 28, 3417–3432 (2000).
Majorek, K. A. et al. The RNase H-like superfamily: new members, comparative structural analysis and evolutionary classification. Nucleic Acids Res. 42, 4160–4179 (2014).
Xiang, G. et al. Evolutionary mining and functional characterization of TnpB nucleases identify efficient miniature genome editors. Nat. Biotechnol. 42, 745–757 (2024).
Makarova, K. S., Wolf, Y. I. & Koonin, E. V. in CRISPR: Biology and Applications (eds Barrangou, R., Sontheimer, E. J. & Marraffini, L. A.) Ch. 2 (Wiley, 2022).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR–Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017).
Nakagawa, R. et al. Cryo-EM structure of the transposon-associated TnpB enzyme. Nature 616, 390–397 (2023).
Sasnauskas, G. et al. TnpB structure reveals minimal functional core of Cas12 nuclease family. Nature 616, 384–389 (2023).
Swarts, D. C., van der Oost, J. & Jinek, M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR–Cas12a. Mol. Cell 66, 221–233 (2017).
Huang, C. J., Adler, B. A. & Doudna, J. A. A naturally DNase-free CRISPR–Cas12c enzyme silences gene expression. Mol. Cell 82, 2148–2160 (2022).
Wiegand, T. et al. TnpB homologues exapted from transposons are RNA-guided transcription factors. Nature 631, 439–448 (2024).
Turchiano, G. et al. Quantitative evaluation of chromosomal rearrangements in gene-edited human stem cells by CAST-seq. Cell Stem Cell 28, 1136–1147 (2021).
Takeda, S. N. et al. Structure of the miniature type V-F CRISPR–Cas effector enzyme. Mol. Cell 81, 558–570 (2021).
Xiao, R., Li, Z., Wang, S., Han, R. & Chang, L. Structural basis for substrate recognition and cleavage by the dimerization-dependent CRISPR–Cas12f nuclease. Nucleic Acids Res. 49, 4120–4128 (2021).
Nety, S. P. et al. The transposon-encoded protein TnpB processes its own mRNA into ωRNA for guided nuclease activity. CRISPR J. 6, 232–242 (2023).
Shmakov, S. et al. Discovery and functional characterization of diverse class 2 CRISPR–Cas systems. Mol. Cell 60, 385–397 (2015).
Kurihara, N. et al. Structure of the type V-C CRISPR–Cas effector enzyme. Mol. Cell 82, 1865–1877 (2022).
Zhang, B. et al. Structural insights into target DNA recognition and cleavage by the CRISPR–Cas12c1 system. Nucleic Acids Res. 50, 11820–11833 (2022).
Li, Z., Zhang, H., Xiao, R., Han, R. & Chang, L. Cryo-EM structure of the RNA-guided ribonuclease Cas12g. Nat. Chem. Biol. 17, 387–393 (2021).
Strecker, J. et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 365, 48–53 (2019).
Sun, A. et al. The compact Casπ (Cas12l) ‘bracelet’ provides a unique structural platform for DNA manipulation. Cell Res. 33, 229–244 (2023).
Harrington, L. B. et al. A scoutRNA is required for some type V CRISPR–Cas systems. Mol. Cell 79, 416–424 (2020).
Liu, J. J. et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature 566, 218–223 (2019).
Liao, C. & Beisel, C. L. The tracrRNA in CRISPR biology and technologies. Annu. Rev. Genet. 55, 161–181 (2021).
Fonfara, I., Richter, H., Bratovič, M., Le Rhun, A. & Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521 (2016).
Zetsche, B. et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 35, 31–34 (2017).
Yan, W. X. et al. Functionally diverse type V CRISPR–Cas systems. Science 363, 88–91 (2019).
Pausch, P. et al. CRISPR–CasΦ from huge phages is a hypercompact genome editor. Science 369, 333–337 (2020).
Al-Shayeb, B. et al. Diverse virus-encoded CRISPR–Cas systems include streamlined genome editors. Cell 185, 4574–4586 (2022).
Zhang, H., Li, Z., Xiao, R. & Chang, L. Mechanisms for target recognition and cleavage by the Cas12i RNA-guided endonuclease. Nat. Struct. Mol. Biol. 27, 1069–1076 (2020).
Karvelis, T. et al. PAM recognition by miniature CRISPR–Cas12f nucleases triggers programmable double-stranded DNA target cleavage. Nucleic Acids Res. 48, 5016–5023 (2020).
Bravo, J. P. K. et al. RNA targeting unleashes indiscriminate nuclease activity of CRISPR–Cas12a2. Nature 613, 582–587 (2023).
Abudayyeh, O. O. et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016).
Abudayyeh, O. O. et al. RNA targeting with CRISPR–Cas13. Nature 550, 280–284 (2017).
Gao, P., Yang, H., Rajashankar, K. R., Huang, Z. & Patel, D. J. Type V CRISPR–Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 26, 901–913 (2016).
Yamano, T. et al. Structural basis for the canonical and non-canonical PAM recognition by CRISPR–Cpf1. Mol. Cell 67, 633–645 (2017).
Xiao, R. et al. Structural basis of target DNA recognition by CRISPR–Cas12k for RNA-guided DNA transposition. Mol. Cell 81, 4457–4466 (2021).
Swarts, D. C. et al. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 21, 743–753 (2014).
Hegge, J. W., Swarts, D. C. & van der Oost, J. Prokaryotic Argonaute proteins: novel genome-editing tools? Nat. Rev. Microbiol. 16, 5–11 (2018).
Swarts, D. C. Making the cut(s): how Cas12a cleaves target and non-target DNA. Biochem. Soc. Trans. 47, 1499–1510 (2019).
Selkova, P. et al. Position of Deltaproteobacteria Cas12e nuclease cleavage sites depends on spacer length of guide RNA. RNA Biol. 17, 1472–1479 (2020).
Lei, C. et al. The CCTL (Cpf1-assisted cutting and Taq DNA ligase-assisted ligation) method for efficient editing of large DNA constructs in vitro. Nucleic Acids Res. 45, e74 (2017).
Strecker, J. et al. Engineering of CRISPR–Cas12b for human genome editing. Nat. Commun. 10, 212 (2019).
Huang, X. et al. Structural basis for two metal-ion catalysis of DNA cleavage by Cas12i2. Nat. Commun. 11, 5241 (2020).
Chen, J. S. et al. CRISPR–Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018).
Harrington, L. B. et al. Programmed DNA destruction by miniature CRISPR–Cas14 enzymes. Science 362, 839–842 (2018).
Li, L. et al. HOLMESv2: a CRISPR–Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synth. Biol. 8, 2228–2237 (2019).
Swarts, D. C. & Jinek, M. Mechanistic insights into the cis- and trans-acting DNase activities of Cas12a. Mol. Cell 73, 589–600 (2019).
Marino, N. D., Pinilla-Redondo, R. & Bondy-Denomy, J. CRISPR–Cas12a targeting of ssDNA plays no detectable role in immunity. Nucleic Acids Res. 50, 6414–6422 (2022).
Dmytrenko, O. et al. Cas12a2 elicits abortive infection through RNA-triggered destruction of dsDNA. Nature 613, 588–594 (2023).
Wendt, K. E., Ungerer, J., Cobb, R. E., Zhao, H. & Pakrasi, H. B. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Micro. Cell Fact. 15, 115 (2016).
Jiang, Y. et al. CRISPR–Cpf1 assisted genome editing of Corynebacterium glutamicum. Nat. Commun. 8, 15179 (2017).
Zhang, Y. et al. Expanding the scope of plant genome engineering with Cas12a orthologs and highly multiplexable editing systems. Nat. Commun. 12, 1944 (2021).
Naduthodi, M. I. S. et al. CRISPR–Cas ribonucleoprotein mediated homology-directed repair for efficient targeted genome editing in microalgae Nannochloropsis oceanica IMET1. Biotechnol. Biofuels 12, 66 (2019).
Wrighton, P. J. et al. Chemically modified AsCas12a guide RNAs improve lipid nanoparticle–mediated in vivo gene editing in different tissues. Presented at The American Society of Gene and Cell Therapy (ASGCT) Annual Meeting, May 7–11 (2024).
Sousa, P. et al. Preclinical development of EDIT301, an autologous cell therapy comprising AsCas12a-RNP modified mobilized peripheral blood-CD34+ cells for the potential treatment of transfusion dependent β thalassemia. Blood 138, 1858 (2021).
Stein, R. A. Molecular scissors are making the cut…in clinical trials: treatments based on various genome editing technologies—CRISPR, TALEN, ZFN, and meganuclease technologies—are starting to reach patients. Genet. Eng. Biotechnol. N. 43, 36–38 (2023).
Hino, T. et al. An AsCas12f-based compact genome-editing tool derived by deep mutational scanning and structural analysis. Cell 186, 4920–4935 (2023).
Bigelyte, G. et al. Miniature type V-F CRISPR–Cas nucleases enable targeted DNA modification in cells. Nat. Commun. 12, 6191 (2021).
Kim, D. Y. et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat. Biotechnol. 40, 94–102 (2022).
Wu, Z. et al. Programmed genome editing by a miniature CRISPR–Cas12f nuclease. Nat. Chem. Biol. 17, 1132–1138 (2021).
Xu, X. et al. Engineered miniature CRISPR–Cas system for mammalian genome regulation and editing. Mol. Cell 81, 4333–4345 (2021).
Chen, W. et al. Cas12n nucleases, early evolutionary intermediates of type V CRISPR, comprise a distinct family of miniature genome editors. Mol. Cell 83, 2768–2780 (2023).
Wang, Y. et al. Guide RNA engineering enables efficient CRISPR editing with a miniature Syntrophomonas palmitatica Cas12f1 nuclease. Cell Rep. 40, 111418 (2022).
Tou, C. J., Orr, B. & Kleinstiver, B. P. Precise cut-and-paste DNA insertion using engineered type V-K CRISPR-associated transposases. Nat. Biotechnol. 41, 968–979 (2023).
Zhang, H. et al. An engineered xCas12i with high activity, high specificity, and broad PAM range. Protein Cell 14, 538–543 (2023).
Chen, Y. et al. Synergistic engineering of CRISPR–Cas nucleases enables robust mammalian genome editing. Innovation 3, 100264 (2022).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Kleinstiver, B. P. et al. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Huang, H. et al. Engineered Cas12a-Plus nuclease enables gene editing with enhanced activity and specificity. BMC Biol. 20, 91 (2022).
Kleinstiver, B. P. et al. Engineered CRISPR–Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol. 37, 276–282 (2019).
Gao, L. et al. Engineered Cpf1 variants with altered PAM specificities. Nat. Biotechnol. 35, 789–792 (2017).
Tóth, E. et al. Improved LbCas12a variants with altered PAM specificities further broaden the genome targeting range of Cas12a nucleases. Nucleic Acids Res. 48, 3722–3733 (2020).
Kim, D. Y. et al. Hypercompact adenine base editors based on transposase B guided by engineered RNA. Nat. Chem. Biol. 18, 1005–1013 (2022).
Ma, E. et al. Improved genome editing by an engineered CRISPR–Cas12a. Nucleic Acids Res. 50, 12689–12701 (2022).
Wu, T. et al. An engineered hypercompact CRISPR–Cas12f system with boosted gene-editing activity. Nat. Chem. Biol. 19, 1384–1393 (2023).
Li, Z. et al. Engineering a transposon-associated TnpB–ωRNA system for efficient gene editing and phenotypic correction of a tyrosinaemia mouse model. Nat. Commun. 15, 831 (2024).
Marquart, K. F. et al. Effective genome editing with an enhanced ISDra2 TnpB system and deep learning-predicted ωRNAs. Nat. Methods https://doi.org/10.1038/s41592-024-02418-z (2024).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Parameshwaran, H. P. et al. The bridge helix of Cas12a imparts selectivity in cis-DNA cleavage and regulates trans-DNA cleavage. FEBS Lett. 595, 892–912 (2021).
Yamano, T. et al. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell 165, 949–962 (2016).
Li, X. et al. Base editing with a Cpf1–cytidine deaminase fusion. Nat. Biotechnol. 36, 324–327 (2018).
Wang, X. et al. Cas12a base editors induce efficient and specific editing with low DNA damage response. Cell Rep. 31, 107723 (2020).
Certo, M. T. et al. Tracking genome engineering outcome at individual DNA breakpoints. Nat. Methods 8, 671–676 (2011).
Wu, W. Y., Lebbink, J. H. G., Kanaar, R., Geijsen, N. & van der Oost, J. Genome editing by natural and engineered CRISPR-associated nucleases. Nat. Chem. Biol. 14, 642–651 (2018).
Anzalone, A. V. et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat. Biotechnol. 40, 731–740 (2022).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Kim, Y. B. et al. A novel mechanistic framework for precise sequence replacement using reverse transcriptase and diverse CRISPR–Cas systems. Preprint at bioRxiv https://doi.org/10.1101/2022.12.13.520319 (2022).
Liang, R. et al. Prime editing using CRISPR–Cas12a and circular RNAs in human cells. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02095-x (2024).
Liu, B. et al. A split prime editor with untethered reverse transcriptase and circular RNA template. Nat. Biotechnol. 40, 1388–1393 (2022).
Meliawati, M., Schilling, C. & Schmid, J. Recent advances of Cas12a applications in bacteria. Appl. Microbiol. Biotechnol. 105, 2981–2990 (2021).
Wu, Y. et al. CAMERS-B: CRISPR/Cpf1 assisted multiple-genes editing and regulation system for Bacillus subtilis. Biotechnol. Bioeng. 117, 1817–1825 (2020).
Schilling, C., Koffas, M. A. G., Sieber, V. & Schmid, J. Novel prokaryotic CRISPR–Cas12a-based tool for programmable transcriptional activation and repression. ACS Synth. Biol. 9, 3353–3363 (2020).
Tak, Y. E. et al. Inducible and multiplex gene regulation using CRISPR–Cpf1-based transcription factors. Nat. Methods 14, 1163–1166 (2017).
Kong, X. et al. Engineered CRISPR–OsCas12f1 and RhCas12f1 with robust activities and expanded target range for genome editing. Nat. Commun. 14, 2046 (2023).
Liang, M. et al. Activating cryptic biosynthetic gene cluster through a CRISPR–Cas12a-mediated direct cloning approach. Nucleic Acids Res. 50, 3581–3592 (2022).
Enghiad, B. et al. Cas12a-assisted precise targeted cloning using in vivo Cre–lox recombination. Nat. Commun. 12, 1171 (2021).
Broughton, J. P. et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 38, 870–874 (2020).
Li, S. Y. et al. CRISPR–Cas12a-assisted nucleic acid detection. Cell Discov. 4, 20 (2018).
Li, S. Y. et al. CRISPR–Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res. 28, 491–493 (2018).
Kaminski, M. M., Abudayyeh, O. O., Gootenberg, J. S., Zhang, F. & Collins, J. J. CRISPR-based diagnostics. Nat. Biomed. Eng. 5, 643–656 (2021).
Teng, F. et al. CDetection: CRISPR–Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 20, 132 (2019).
Wang, Z. & Zhong, C. Cas12c-DETECTOR: a specific and sensitive Cas12c-based DNA detection platform. Int. J. Biol. Macromol. 193, 441–449 (2021).
Rananaware, S. R. et al. Programmable RNA detection with CRISPR–Cas12a. Nat. Commun. 14, 5409 (2023).
Zhou, S. et al. CRISPR/Cas12a-drived fluorescent and electrochemical signal-off/on dual-mode biosensors for ultrasensitive detection of EGFR 19del mutation. Sensor Actuat. B Chem. 392, 134034 (2023).
Klompe, S. E., Vo, P. L. H., Halpin-Healy, T. S. & Sternberg, S. H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 571, 219–225 (2019).
Peters, J. E., Makarova, K. S., Shmakov, S. & Koonin, E. V. Recruitment of CRISPR–Cas systems by Tn7-like transposons. Proc. Natl Acad. Sci. USA 114, E7358–E7366 (2017).
Park, J. U. et al. Structures of the holo CRISPR RNA-guided transposon integration complex. Nature 613, 775–782 (2023).
Querques, I., Schmitz, M., Oberli, S., Chanez, C. & Jinek, M. Target site selection and remodelling by type V CRISPR-transposon systems. Nature 599, 497–502 (2021).
Altae-Tran, H. et al. Uncovering the functional diversity of rare CRISPR–Cas systems with deep terascale clustering. Science 382, eadi1910 (2023).
Durrant, M. G. et al. Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome. Nat. Biotechnol. 41, 488–499 (2023).
Chen, F. et al. Multiplexed base editing through Cas12a variant-mediated cytosine and adenine base editors. Commun. Biol. 5, 1163 (2022).
Yarnall, M. T. N. et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat. Biotechnol. 41, 500–512 (2023).
Vialetto, E. et al. Systematic interrogation of CRISPR antimicrobials in Klebsiella pneumoniae reveals nuclease-, guide- and strain-dependent features influencing antimicrobial activity. Nucleic Acids Res. 52, 6079–6091 (2024).
Ding, X. et al. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR–Cas12a assay. Nat. Commun. 11, 4711 (2020).
Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science 379, eadd8643 (2023).
Xin, C. et al. Comprehensive assessment of miniature CRISPR–Cas12f nucleases for gene disruption. Nat. Commun. 13, 5623 (2022).
Li, S. et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 13, 5561 (2022).
Villiger, L. et al. CRISPR technologies for genome, epigenome and transcriptome editing. Nat. Rev. Mol. Cell Biol. 25, 464–487 (2024).
Wong, C. UK first to approve CRISPR treatment for diseases: what you need to know. Nature 623, 676–677 (2023).
Longhurst, H. J. et al. CRISPR–Cas9 in vivo gene editing of KLKB1 for hereditary angioedema. N. Engl. J. Med. 390, 432–441 (2024).
O’Leary, K. Gene-editing breakthrough for a rare hereditary disorder. Nat. Med. https://doi.org/10.1038/d41591-024-00008-2 (2024).
Chew, W. L. Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1408 (2018).
Wagner, D. L. et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 25, 242–248 (2019).
Ferdosi, S. R. et al. Multifunctional CRISPR–Cas9 with engineered immunosilenced human T cell epitopes. Nat. Commun. 10, 1842 (2019).
Burstein, D. et al. New CRISPR–Cas systems from uncultivated microbes. Nature 542, 237–241 (2017).
Yang, H. & Patel, D. J. CasX: a new and small CRISPR gene-editing protein. Cell Res. 29, 345–346 (2019).
Carabias, A. et al. Structure of the mini-RNA-guided endonuclease CRISPR–Cas12j3. Nat. Commun. 12, 4476 (2021).
Park, J. U. et al. Structural basis for target site selection in RNA-guided DNA transposition systems. Science 373, 768–774 (2021).
Urbaitis, T. et al. A new family of CRISPR-type V nucleases with C-rich PAM recognition. EMBO Rep. 23, e55481 (2022).
Acknowledgements
This research is supported by research grants of the Dutch Research Council (NWO; BBOL-737.016.005, Spinoza SPI 93–537 and Gravitation 024.003.019) and the European Research Council (ERC-AdG-834279).
Author information
Authors and Affiliations
Contributions
W.Y.W., B.A.-P. and J.v.d.O. conceptualized the review and contributed to the writing of the manuscript. W.Y.W. and B.A.-P. prepared the visual elements, including figure and table design.
Corresponding authors
Ethics declarations
Competing interests
Several CRISPR–Cas-related patent applications have been filed related to this work, with J.v.d.O. and W.Y.W. as inventors. J.v.d.O. is cofounder and scientific adviser of NTrans Technologies and adviser of Scope Biosciences and Hudson River Biotechnology. B.A-P. declares no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Chase Beisel and Haoyi Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, W.Y., Adiego-Pérez, B. & van der Oost, J. Biology and applications of CRISPR–Cas12 and transposon-associated homologs. Nat Biotechnol 42, 1807–1821 (2024). https://doi.org/10.1038/s41587-024-02485-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41587-024-02485-9
This article is cited by
-
Engineering hypercompact IscB nucleases for efficient and versatile genome editing in rice
Genome Biology (2026)
-
Phage-associated Cas12p nucleases require binding to bacterial thioredoxin for activation and cleavage of target DNA
Nature Microbiology (2026)
-
RNA-triggered Cas12a3 cleaves tRNA tails to execute bacterial immunity
Nature (2026)
-
Structural basis for target DNA cleavage and guide RNA processing by CRISPR-Casλ2
Communications Biology (2025)
-
Epigenetic editing: from concept to clinic
Nature Reviews Drug Discovery (2025)