Abstract
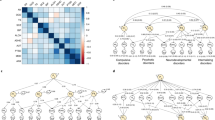
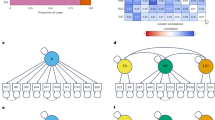
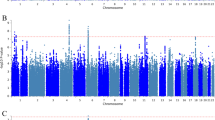
Differences in the patterning of genetic sharing between groups of individuals may arise from biological pathways, social mechanisms, phenotyping and ascertainment. We expand genomic structural equation modeling to allow for testing genomic structural invariance (GSI), that is, the formal comparison of multivariate genetic architecture across groups. We apply GSI to compare the autosomal multivariate genetic architecture of eight psychiatric disorders spanning three factors (psychotic, neurodevelopmental and internalizing) between cisgender males and females. We find that the genetic factor structure is largely similar across sex, permitting meaningful comparisons of associations at the level of the factors. However, in females, problematic alcohol use and posttraumatic stress disorder loaded more strongly on the internalizing factor, while the neurodevelopmental disorder factor exhibited weaker genetic correlations with the other factors. Four phenotypes (educational attainment, insomnia, smoking and deprivation) showed significant, albeit small, sex-differentiated associations with the psychotic factor. As genome-wide association study samples grow and diversify, GSI will become increasingly valuable for comparing multivariate genetic architecture across groups.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Disorder-level summary statistics from previously published GWAS can be found at the citations in Table 1. Factor-level, sex-stratified summary statistics and sex-stratified GWAS summary statistics for PTSD and alcohol problems produced for this study are available at https://osf.io/spg7f/ (ref. 59); see the Supplementary Note for complete information on these phenotypes. The sex-stratified eight-disorder LDSC object that can be used to reproduce the results is available at https://osf.io/pd4fx (ref. 59).
Code availability
A tutorial for conducting GSI analyses and estimating localSRMD is available at https://rpubs.com/tedooooooooooo/localsrmd (ref. 59). The R code for all genomic SEM analyses can be found at https://osf.io/wya8p (ref. 59).
References
Grotzinger, A. D. et al. Genetic architecture of 11 major psychiatric disorders at biobehavioral, functional genomic, and molecular genetic levels of analysis. Nat. Genet. 54, 548–559 (2022).
Lee, P. H. et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482 (2019).
Lee, P. H., Feng, Y.-C. A. & Smoller, J. W. Pleiotropy and cross-disorder genetics among psychiatric disorders. Biol. Psychiatry 89, 20–31 (2021).
Khramtsova, E. A. et al. Quality control and analytic best practices for testing genetic models of sex differences in large populations. Cell 186, 2044–2061 (2023).
Mostafavi, H. et al. Variable prediction accuracy of polygenic scores within an ancestry group. eLife 9, e48376 (2020).
Grotzinger, A. D. et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat. Hum. Behav. 3, 513–525 (2019).
Abel, K. M., Drake, R. & Goldstein, J. M. Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428 (2010).
Dohrenwend, B. P. & Dohrenwend, B. S. Sex differences and psychiatric disorders. AJS 81, 1447–1454 (1976).
Merikangas, A. K. & Almasy, L. Using the tools of genetic epidemiology to understand sex differences in neuropsychiatric disorders. Genes Brain Behav. 19, e12660 (2020).
Bernabeu, E. et al. Sex differences in genetic architecture in the UK Biobank. Nat. Genet. 53, 1283–1289 (2021).
Blokland, G. A. M. et al. Sex-dependent shared and non-shared genetic architecture, across mood and psychotic disorders. Biol. Psychiatry 91, 102–117 (2022).
Duncan, L. E. et al. Largest GWAS of PTSD (N = 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 23, 666–673 (2018).
Martin, J. et al. Examining sex-differentiated genetic effects across neuropsychiatric and behavioral traits. Biol. Psychiatry 89, 1127–1137 (2021).
Silveira, P. P., Pokhvisneva, I., Howard, D. M. & Meaney, M. J. A sex-specific genome-wide association study of depression phenotypes in UK Biobank. Mol. Psychiatry 28, 2469–2479 (2023).
Zhu, C. et al. Amplification is the primary mode of gene-by-sex interaction in complex human traits. Cell Genom. 3, 100297 (2023).
Khramtsova, E. A., Davis, L. K. & Stranger, B. E. The role of sex in the genomics of human complex traits. Nat. Rev. Genet. 20, 173–190 (2019).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Lorenzo-Seva, U. & Ten Berge, J. M. Tucker’s congruence coefficient as a meaningful index of factor similarity. Methodology 2, 57–64 (2006).
Funder, D. C. & Ozer, D. J. Evaluating effect size in psychological research: sense and nonsense. Adv. Methods Pract. Psychol. Sci. 2, 156–168 (2019).
Van Der Sluis, S., Dolan, C. V. & Stoel, R. D. A note on testing perfect correlations in SEM. Struct. Equ. Model. 12, 551–577 (2005).
Gogos, A., Ney, L. J., Seymour, N., Van Rheenen, T. E. & Felmingham, K. L. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: are gonadal hormones the link? Br. J. Pharmacol. 176, 4119–4135 (2019).
Sinnott-Armstrong, N., Naqvi, S., Rivas, M. & Pritchard, J. K. GWAS of three molecular traits highlights core genes and pathways alongside a highly polygenic background. eLife 10, e58615 (2021).
Harden, K. P. Genetic influences on adolescent sexual behavior: why genes matter for environmentally oriented researchers. Psychol. Bull. 140, 434–465 (2014).
Moore, S. R., Harden, K. P. & Mendle, J. Pubertal timing and adolescent sexual behavior in girls. Dev. Psychol. 50, 1734–1745 (2014).
Lake, A. M., Goleva, S. B., Samuels, L. R., Carpenter, L. M. & Davis, L. K. Sex differences in health conditions associated with sexual assault in a large hospital population. Complex Psychiatry 8, 80–89 (2023).
Tolin, D. F. & Foa, E. B. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 132, 959–992 (2006).
Bourgeois, C., Lecomte, T. & Daigneault, I. Psychotic disorders in sexually abused youth: a prospective matched-cohort study. Schizophr. Res. 199, 123–127 (2018).
Miller, M. W. & Resick, P. A. Internalizing and externalizing subtypes in female sexual assault survivors: implications for the understanding of complex PTSD. Behav. Ther. 38, 58–71 (2007).
Lai, M. C. & Baron-Cohen, S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2, 1013–1027 (2015).
Rødgaard, E. M., Jensen, K., Miskowiak, K. W. & Mottron, L. Childhood diagnoses in individuals identified as autistics in adulthood. Mol. Autism 12, 73 (2021).
Bilghese, M. et al. A general approach to adjusting genetic studies for assortative mating. Preprint at bioRxiv https://doi.org/10.1101/2023.09.01.555983 (2023).
Border, R. et al. Cross-trait assortative mating is widespread and inflates genetic correlation estimates. Science 378, 754–761 (2022).
Grotzinger, A. D. & Keller, M. C. Potential bias in genetic correlations. Science 378, 709–710 (2022).
Hatoum, A. S. et al. Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nat. Ment. Health 1, 210–223 (2023).
Brown, B. C., Ye, C. J., Price, A. L. & Zaitlen, N. Transethnic genetic-correlation estimates from summary statistics. Am. J. Hum. Genet. 99, 76–88 (2016).
Shi, H. et al. Population-specific causal disease effect sizes in functionally important regions impacted by selection. Nat. Commun. 12, 1098 (2021).
Turley, P. et al. Multi-ancestry meta-analysis yields novel genetic discoveries and ancestry-specific associations. Preprint at bioRxiv https://doi.org/10.1101/2021.04.23.441003 (2021).
Walters, R. K. et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci. 21, 1656–1669 (2018).
Mallard, T. T. et al. Item-level genome-wide association study of the alcohol use disorders identification test in three population-based cohorts. Am. J. Psychiatry 179, 58–70 (2022).
Martin, J. et al. Sex‐specific manifestation of genetic risk for attention deficit hyperactivity disorder in the general population. J. Child Psychol. Psychiatry 59, 908–916 (2018).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Maihofer, A. X. et al. Enhancing discovery of genetic variants for posttraumatic stress disorder through integration of quantitative phenotypes and trauma exposure information. Biol. Psychiatry 91, 626–636 (2022).
Coleman, J. R. I. et al. The genetics of the mood disorder spectrum: genome-wide association analyses of more than 185,000 cases and 439,000 controls. Biol. Psychiatry 88, 169–184 (2020).
De Vlaming, R., Johannesson, M., Magnusson, P. K., Ikram, M. A. & Visscher, P. M. Equivalence of LD-score regression and individual-level-data methods. Preprint at bioRxiv https://doi.org/10.1101/211821 (2017).
Grotzinger, A. D., de la Fuente, J., Privé, F., Nivard, M. G. & Tucker-Drob, E. M. Pervasive downward bias in estimates of liability scale heritability in GWAS meta-analysis: a simple solution. Biol. Psychiatry 93, 29–36 (2023).
Evans, L. M. et al. Comparison of methods that use whole genome data to estimate the heritability and genetic architecture of complex traits. Nat. Genet. 50, 737–745 (2018).
Horn, J. L. & McArdle, J. J. A practical and theoretical guide to measurement invariance in aging research. Exp. Aging Res. 18, 117–144 (1992).
Putnick, D. L. & Bornstein, M. H. Measurement invariance conventions and reporting: the state of the art and future directions for psychological research. Dev. Rev. 41, 71–90 (2016).
Vandenberg, R. J. & Lance, C. E. A review and synthesis of the measurement invariance literature: suggestions, practices, and recommendations for organizational research. Organ. Res. Methods 3, 4–70 (2000).
Kenny, D. A. Measuring model fit. http://davidakenny.net/cm/fit.htm (2014).
Hu, L. T. & Bentler, P. M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model. 6, 1–55 (1999).
Drasgow, F. Scrutinizing psychological tests: measurement equivalence and equivalent relations with external variables are the central issues. Psychol. Bull. 95, 134–135 (1984).
Meredith, W. Measurement invariance, factor analysis and factorial invariance. Psychometrika 58, 525–543 (1993).
Byrne, B. M., Shavelson, R. J. & Muthén, B. Testing for the equivalence of factor covariance and mean structures: the issue of partial measurement invariance. Psychol. Bull. 105, 456–466 (1989).
Chen, F. F. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct. Equ. Model. 14, 464–504 (2007).
Shi, D., Maydeu-Olivares, A. & DiStefano, C. The relationship between the standardized root mean square residual and model misspecification in factor analysis models. Multivariate Behav. Res. 53, 676–694 (2018).
Cheung, G. W. & Rensvold, R. B. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct. Equ. Model. 9, 233–255 (2002).
Schwaba, T., Grotzinger, A. D., Nivard, M. G., Davis, L. & Tucker-Drob, E. M. Testing sex differences in the multivariate genetic architecture of psychiatric disorders. OSF https://doi.org/10.17605/OSF.IO/SPG7F (2022).
Lakens, D., Scheel, A. M. & Isager, P. M. Equivalence testing for psychological research: a tutorial. Adv. Meth. Pract. Psychol. 1, 259–269 (2018).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Acknowledgements
This work was supported by a National Institutes of Health (NIH) grant no. R01MH120219. E.M.T.-D. is a faculty associate of the Population Research Center and the Center on Aging and Population Sciences at the University of Texas at Austin, which are supported by NIH grant nos. P2CHD042849 and P30AG066614, respectively.
Author information
Authors and Affiliations
Contributions
T.S. and E.M.T.-D. designed the study and wrote the manuscript. T.S. conducted the multivariate analyses. T.T.M. and A.X.M. contributed new genome-wide analyses. T.S., E.M.T.-D., M.R. and M.G.N. conceptualized the statistical tests. L.K.D. consulted on the role of sex as a biological variable. T.T.M., A.X.M., M.R., P.H.L., J.W.S., L.K.D., M.G.N. and A.D.G. provided manuscript feedback and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Jonathan Coleman, Frank Wendt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary localSRMD guide, Figs. 1–15 and Discussion.
Supplementary Table 1
Supplementary Tables 1–18.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schwaba, T., Mallard, T.T., Maihofer, A.X. et al. Comparison of the multivariate genetic architecture of eight major psychiatric disorders across sex. Nat Genet 57, 583–590 (2025). https://doi.org/10.1038/s41588-025-02093-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41588-025-02093-6