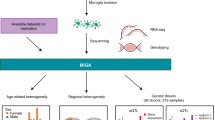
Abstract
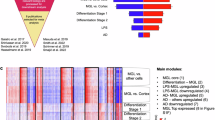
Microglia, the innate immune cells of the central nervous system, have been genetically implicated in multiple neurodegenerative diseases. Mapping the genetics of gene expression in human microglia has identified several loci associated with disease-associated genetic variants in microglia-specific regulatory elements. However, identifying genetic effects on splicing is challenging because of the use of short sequencing reads. Here, we present the isoform-centric microglia genomic atlas (isoMiGA), which leverages long-read RNA sequencing to identify 35,879 novel microglia isoforms. We show that these isoforms are involved in stimulation response and brain region specificity. We then quantified the expression of both known and novel isoforms in a multi-ancestry meta-analysis of 555 human microglia short-read RNA sequencing samples from 391 donors, and found associations with genetic risk loci in Alzheimer’s and Parkinson’s disease. We nominate several loci that may act through complex changes in isoform and splice-site usage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Datasets used in this study are publicly available as follows: long-read data at https://www.synapse.org/Synapse:syn64371963, Gaffney short-read cohort at https://ega-archive.org/datasets/EGAD00001005736, Raj short-read microglia samples at https://dss.niagads.org/datasets/ng00105/, Roussos short-read batch 1 at https://doi.org/10.7303/syn26207321, Roussos short-read batch 2 at https://www.synapse.org/Synapse:syn25671146, iPS-derived microglia short-read at Gene Expression Omnibus GSE240907, novel isoform GTF and FASTA via Zenodo at https://zenodo.org/record/8290956 (ref. 93), count matrices for all cohorts via Zenodo at https://doi.org/10.5281/zenodo.8291210 (ref. 94), all QTL summary statistics via Zenodo at https://zenodo.org/record/8250771 (ref. 95), Database of Conjoined Genes at http://metasystems.riken.jp/conjoing/, AD GWAS summary statistics48 at https://www.ebi.ac.uk/gwas/studies/GCST90027158, AD GWAS summary statistics2 at https://www.niagads.org/datasets/ng00036, PD GWAS summary statistics49 at https://research.23andme.com/collaborate/, schizophrenia GWAS summary statistics (Trubetskoy) at https://pgc.unc.edu/for-researchers/download-results/, microglia caQTLs15 at https://doi.org/10.7303/syn26207321 and bulk brain eQTLs and sQTLs (CommonMind) at https://www.synapse.org/#!Synapse:syn2759792. Source data are provided with this paper.
Code availability
The genotype quality control pipeline is available via Zenodo at https://doi.org/10.5281/zenodo.13864727 (ref. 96). The long-read RNA-seq pipeline is available via Zenodo at https://doi.org/10.5281/zenodo.13864731 (ref. 97). The QTL preparation and meta-analysis pipeline is available via Zenodo at https://doi.org/10.5281/zenodo.13864735 (ref. 98). The colocalization and MESC pipelines are available via Zenodo at https://doi.org/10.5281/zenodo.13864729 (ref. 99). All code used to produce analysis for figures is available via Zenodo at https://doi.org/10.5281/zenodo.13864720 (ref. 100).
References
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 (2013).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458 (2013).
Raj, T. et al. CD33: increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer’s disease susceptibility. Hum. Mol. Genet. 23, 2729–2736 (2014).
Raj, T. et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344, 519–523 (2014).
Efthymiou, A. G. & Goate, A. M. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 12, 43 (2017).
Huang, K.-L. et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat. Neurosci. 20, 1052–1061 (2017).
Schwartzentruber, J. et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat. Genet. 53, 392–402 (2021).
Andersen, M. S. et al. Heritability enrichment implicates microglia in Parkinson’s disease pathogenesis. Ann. Neurol. 89, 942–951 (2021).
Novikova, G. et al. Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat. Commun. 12, 1610 (2021).
Navarro, E. et al. Dysregulation of mitochondrial and proteolysosomal genes in Parkinson’s disease myeloid cells. Nat. Aging 1, 850–863 (2021).
Nott, A. et al. Brain cell type-specific enhancer–promoter interactome maps and disease-risk association. Science 366, 1134–1139 (2019).
Corces, M. R. et al. Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nat. Genet. 52, 1158–1168 (2020).
Young, A. M. H. et al. A map of transcriptional heterogeneity and regulatory variation in human microglia. Nat. Genet. 53, 861–868 (2021).
Lopes, K. P. et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat. Genet. 54, 4–17 (2022).
Kosoy, R. et al. Genetics of the human microglia regulome refines Alzheimer’s disease risk loci. Nat. Genet. 54, 1145–1154 (2022).
Langston, R. G. et al. Association of a common genetic variant with Parkinson’s disease is mediated by microglia. Sci. Transl. Med. 14, eabp8869 (2022).
Bryois, J. et al. Cell-type-specific cis-eQTLs in eight human brain cell types identify novel risk genes for psychiatric and neurological disorders. Nat. Neurosci. 25, 1104–1112 (2022).
Schilder, B. M. & Raj, T. Fine-mapping of Parkinson’s disease susceptibility loci identifies putative causal variants. Hum. Mol. Genet. 31, 888–900 (2022).
Fujita, M. et al. Cell subtype-specific effects of genetic variation in the Alzheimer’s disease brain. Nat. Genet. 56, 605–614 (2024).
Li, Y. I. et al. RNA splicing is a primary link between genetic variation and disease. Science 352, 600–604 (2016).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Mittleman, B. E. et al. Alternative polyadenylation mediates genetic regulation of gene expression. eLife 9, e57492 (2020).
Alasoo, K. et al. Genetic effects on promoter usage are highly context-specific and contribute to complex traits. eLife 8, e41673 (2019).
Li, Y. I. et al. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 50, 151–158 (2018).
Raj, T. et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat. Genet. 50, 1584–1592 (2018).
Monlong, J., Calvo, M., Ferreira, P. G. & Guigó, R. Identification of genetic variants associated with alternative splicing using sQTLseekeR. Nat. Commun. 5, 4698 (2014).
Qi, T. et al. Genetic control of RNA splicing and its distinct role in complex trait variation. Nat. Genet. 54, 1355–1363 (2022).
Zhang, Y. et al. Regional variation of splicing QTLs in human brain. Am. J. Hum. Genet. 107, 196–210 (2020).
Zhang, D. et al. Incomplete annotation has a disproportionate impact on our understanding of Mendelian and complex neurogenetic disorders. Sci. Adv. 6, eaay8299 (2020).
Sharon, D., Tilgner, H., Grubert, F. & Snyder, M. A single-molecule long-read survey of the human transcriptome. Nat. Biotechnol. 31, 1009–1014 (2013).
Castaldi, P. J., Abood, A., Farber, C. R. & Sheynkman, G. M. Bridging the splicing gap in human genetics with long-read RNA sequencing: finding the protein isoform drivers of disease. Hum. Mol. Genet. 31, R123–R136 (2022).
Leung, S. K. et al. Full-length transcript sequencing of human and mouse cerebral cortex identifies widespread isoform diversity and alternative splicing. Cell Rep. 37, 110022 (2021).
Glinos, D. A. et al. Transcriptome variation in human tissues revealed by long-read sequencing. Nature 608, 353–359 (2022).
Abood, A. et al. Long-read proteogenomics to connect disease-associated sQTLs to the protein isoform effectors of disease. Am. J. Hum. Genet. 111, 1914–1931 (2024).
Shumate, A., Wong, B., Pertea, G. & Pertea, M. Improved transcriptome assembly using a hybrid of long and short reads with StringTie. PLoS Comput. Biol. 18, e1009730 (2022).
Prakash, T. et al. Expression of conjoined genes: another mechanism for gene regulation in eukaryotes. PLoS ONE 5, e13284 (2010).
Bampton, A. et al. HnRNP K mislocalisation is a novel protein pathology of frontotemporal lobar degeneration and ageing and leads to cryptic splicing. Acta Neuropathol. 142, 609–627 (2021).
Kiianitsa, K. et al. Novel TREM2 splicing isoform that lacks the V-set immunoglobulin domain is abundant in the human brain. J. Leukoc. Biol. 110, 829–837 (2021).
Shaw, B. C. et al. An alternatively spliced TREM2 isoform lacking the ligand binding domain is expressed in human brain. J. Alzheimers Dis. 87, 1647–1657 (2022).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
van der Poel, M. et al. Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 10, 1139 (2019).
Navarro, E. et al. LRRK2 G2019S variant is associated with transcriptional changes in Parkinson’s disease human myeloid cells under proinflammatory environment. Preprint at bioRxiv https://doi.org/10.1101/2024.05.27.594821 (2024).
Parikh, I., Fardo, D. W. & Estus, S. Genetics of PICALM expression and Alzheimer’s disease. PLoS ONE 9, e91242 (2014).
Zeng, B. et al. Multi-ancestry eQTL meta-analysis of human brain identifies candidate causal variants for brain-related traits. Nat. Genet. 54, 161–169 (2022).
Trincado, J. L. et al. SUPPA2: fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol. 19, 40 (2018).
Yao, D. W., O’Connor, L. J., Price, A. L. & Gusev, A. Quantifying genetic effects on disease mediated by assayed gene expression levels. Nat. Genet. 52, 626–633 (2020).
Li, Y. I., Wong, G., Humphrey, J. & Raj, T. Prioritizing Parkinson’s disease genes using population-scale transcriptomic data. Nat. Commun. 10, 994 (2019).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Andreone, B. J. et al. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat. Neurosci. 23, 927–938 (2020).
Sims, R. et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384 (2017).
Jaganathan, K. et al. Predicting splicing from primary sequence with deep learning. Cell 176, 535–548.e24 (2019).
Zeng, T. & Li, Y. I. Predicting RNA splicing from DNA sequence using Pangolin. Genome Biol. 23, 103 (2022).
Kerimov, N. et al. eQTL Catalogue 2023: new datasets, X chromosome QTLs, and improved detection and visualisation of transcript-level QTLs. PLoS Genet. 19, e1010932 (2023).
Schwartzentruber, J. et al. Molecular and functional variation in iPSC-derived sensory neurons. Nat. Genet. 50, 54–61 (2018).
Joglekar, A. et al. A spatially resolved brain region- and cell type-specific isoform atlas of the postnatal mouse brain. Nat. Commun. 12, 463 (2021).
Volden, R. & Vollmers, C. Single-cell isoform analysis in human immune cells. Genome Biol. 23, 47 (2022).
Al’Khafaji, A. M. et al. High-throughput RNA isoform sequencing using programmed cDNA concatenation. Nat. Biotechnol. 42, 582–586 (2024).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Garrido-Martín, D., Borsari, B., Calvo, M., Reverter, F. & Guigó, R. Identification and analysis of splicing quantitative trait loci across multiple tissues in the human genome. Nat. Commun. 12, 727 (2021).
Fair, B. et al. Global impact of unproductive splicing on human gene expression. Nat. Genet. 56, 1851–1861 (2024).
Mu, Z. et al. The impact of cell type and context-dependent regulatory variants on human immune traits. Genome Biol. 22, 122 (2021).
Mattei, D. et al. Enzymatic dissociation induces transcriptional and proteotype bias in brain cell populations. Int. J. Mol. Sci. 21, 7944 (2020).
Pardo-Palacios, F. J. et al. Systematic assessment of long-read RNA-seq methods for transcript identification and quantification. Nat. Methods 21, 1349–1363 (2024).
Inamo, J. et al. Long-read sequencing for 29 immune cell subsets reveals disease-linked isoforms. Nat. Commun. 15, 4285 (2024).
Wang, X. et al. Detection of proteome diversity resulted from alternative splicing is limited by trypsin cleavage specificity. Mol. Cell. Proteomics 17, 422–430 (2018).
Cooper, Y. A. et al. Functional regulatory variants implicate distinct transcriptional networks in dementia. Science 377, eabi8654 (2022).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Kovaka, S. et al. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 20, 278 (2019).
Tardaguila, M. et al. SQANTI: extensive characterization of long-read transcript sequences for quality control in full-length transcriptome identification and quantification. Genome Res. 28, 396–411 (2018).
Roy, K. R. & Chanfreau, G. F. Robust mapping of polyadenylated and non-polyadenylated RNA 3′ ends at nucleotide resolution by 3′-end sequencing. Methods 176, 4–13 (2020).
Chen, Y. et al. Context-aware transcript quantification from long-read RNA-seq data with Bambu. Nat. Methods 20, 1187–1195 (2023).
Prjibelski, A. D. et al. Accurate isoform discovery with IsoQuant using long reads. Nat. Biotechnol. 41, 915–918 (2023).
Pertea, G. & Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res. 9, ISCB Comm J-304 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Biotechnol. 14, 417–419 (2017).
Soneson, C. Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. https://doi.org/10.12688/f1000research.7563.1 (2015).
Hoffman, G. E. & Roussos, P. Dream: powerful differential expression analysis for repeated measures designs. Bioinformatics 37, 192–201 (2021).
Gilis, J., Vitting-Seerup, K., Van den Berge, K. & Clement, L. satuRn: scalable analysis of differential transcript usage for bulk and single-cell RNA-sequencing applications. F1000Res. 10, 374 (2021).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Taylor-Weiner, A. et al. Scaling computational genomics to millions of individuals with GPUs. Genome Biol. 20, 228 (2019).
Stegle, O., Parts, L., Piipari, M., Winn, J. & Durbin, R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 7, 500 (2012).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Storey, J. D. The positive false discovery rate: a Bayesian interpretation and the q-value. Ann. Stat. 31, 2013–2035 (2003).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Cotto, K. C. et al. Integrated analysis of genomic and transcriptomic data for discovery of splicing variants in cancer. Nat. Commun. 14, 1589 (2023).
Degner, J. F. et al. DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482, 390–394 (2012).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Myers, T. A., Chanock, S. J. & Machiela, M. J. LDlinkR: an R package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front. Genet. 11, 157 (2020).
Wallace, C. A more accurate method for colocalisation analysis allowing for multiple causal variants. PLoS Genet. 17, e1009440 (2021).
Weissbrod, O. et al. Functionally informed fine-mapping and polygenic localization of complex trait heritability. Nat. Genet. 52, 1355–1363 (2020).
Humphrey, J. & Raj, T. isoMiGA: Long-read isoforms discovered in human microglia. Zenodo https://doi.org/10.5281/zenodo.8290955 (2023).
Humphrey, J. & Raj, T. isoMiGA: Isoform and gene-level counts and TPM in short-read human microglia. Zenodo https://doi.org/10.5281/zenodo.8291210 (2023).
Humphrey, J. & Raj, T. isoMIGA: Expression and Splicing QTL Summary Statistics in Human Microglia. Zenodo https://doi.org/10.5281/zenodo.8250770 (2023).
Humphrey, J. & Muller, B. RajLabMSSM/Genotype_QC_Pipeline_2.0: publication. Zenodo https://doi.org/10.5281/zenodo.13864726 (2024).
Humphrey, J. RajLabMSSM/isoseq-Pipeline: publication. Zenodo https://doi.org/10.5281/zenodo.13864730 (2024).
Humphrey, J., Kailash, B. P., Brophy, E. & Naito, T. RajLabMSSM/mmQTL-Pipeline: publication Zenodo https://doi.org/10.5281/zenodo.13864734 (2024).
Humphrey, J., Kailash, B. P. & Cuddleston, W. RajLabMSSM/downstream-QTL: publication. Zenodo https://doi.org/10.5281/zenodo.13864728 (2024).
Humphrey, J. RajLabMSSM/isoMiGA: publication version. Zenodo https://doi.org/10.5281/zenodo.13864719 (2024).
Acknowledgements
We thank the patients and families who donated material for these studies. We thank E. Tseng of Pacific Biosciences who provided important critical feedback on long-read analysis. This study was supported by the following National Institutes of Health grant nos: NIA U01-AG058635, NIA R21-AG063130, NIA R01-AG054005, NIA U01-AG068880, NIA RF1-AG065926, NIA R56-AG055824, NIA P30-AG066514, NINDS U54-NS123743 and NINDS R01-NS116006 to T.R., J.H., E.B., D.M., E.C. and B.Z.M. NIA U01-AG058635, NIA R01-AG067025, NIA R01-AG082185, NIA R01-AG050986, NIA R01-AG065582, NIMH R01-MH125246 and NINDS U01-NS125580 to P.R., R.K., B.Z., S.P.K., S.A., Z.S., G.E.H. and J.F.F. NIA U01-AG058635 to A.M.G., A.M. and A.G.E. This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai and supported by the Clinical and Translational Science Awards (CTSA) grant no. UL1TR004419 from the National Center for Advancing Translational Sciences. Research reported in this paper was supported by the Office of Research Infrastructure of the National Institutes of Health under award numbers S10OD026880 and S10OD030463. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
The study was conceived by J.H., P.R. and T.R. Data analysis was led by J.H., with contributions from E.B., R.K., B.Z., G.E.H., A.R., T.N., B.Z.M. and C.-F.T. Data generation was performed by E.C., D.M., A.G.E., E.N., A.A., G.J.L.J.S., C.D.S., V.F.-A., A.M., S.P.K., S.A., P.M., K.P., R.B.K., Z.S., N.F., C.-F.T., M.A.G., M.E.M., V.L.P., K.K.W., T.S. and J.F.F. Work was supervised by G.E.H., T.L., L.D.d.W., R.S., A.M.G., J.F.F., D.A.B., V.H., P.R. and T.R. The paper was written by J.H., with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The following authors wish to disclose their industry relations: A.M.G. is a scientific advisory board member for Genentech and Muna Therapeutics; R.S. is currently a paid consultant and equity holder at GeneDx; N.F. is currently an employee of Pacific Biosciences; B.Z.M. is currently an employee of Abbvie; A.G.E. is an employee of BlueRock Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Kaur Alasoo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Results, Methods and Figs. 1–14.
Supplementary Tables
Supplementary Tables 1–13.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Humphrey, J., Brophy, E., Kosoy, R. et al. Long-read RNA sequencing atlas of human microglia isoforms elucidates disease-associated genetic regulation of splicing. Nat Genet 57, 604–615 (2025). https://doi.org/10.1038/s41588-025-02099-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41588-025-02099-0
This article is cited by
-
Genetics of PLCG2 expression and splicing relative to Alzheimer’s disease risk
Molecular Neurodegeneration Advances (2025)
-
Elucidating the coordination of RNA processing using short-read and long-read RNA-sequencing methods
Nature Reviews Molecular Cell Biology (2025)