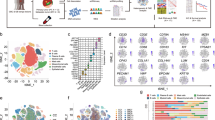
Abstract
Gallbladder carcinoma (GBC) is the most aggressive biliary tract cancer and is associated with a high mortality rate. Treatment of GBC faces therapeutic challenges owing to the elusive nature of in situ drivers within the local tumor microenvironment that drive its progression. Here, we created a single-cell atlas of 1,117,245 cells and a mutational landscape from 102 patients, which unveiled spatial–temporal characterizations of cellular constitutions, spatial interplays and molecular functions, and generalized five local ecosystems stratifying clinical outcomes. An integrated epithelial program, AI-EPI, combined with spatial transcriptome analysis, revealed the concurrent localization of a highly malignant tumor subtype (GM16) and AREG+ T cell, B cell, dendritic cell and macrophage subtypes within the pro-metastatic niche of primary adenocarcinomas. In vitro and in vivo experiments suggest that in addition to promoting metastasis, AREG facilitates CXCL5 expression in tumor cells through EGFR–pERK–EGR1 signaling, leading to increased neutrophil infiltration and impeding the effectiveness of immunotherapy. Our study provides a spatial–temporal landscape of the GBC microenvironment and sheds light on potential strategies for preventing immunotherapy resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw sequencing data have been deposited in two publicly accessible repositories: the CNGB Sequence Archive (CNSA)69 of China National GeneBank DataBase (CNGBdb) under accession number CNP0004660, accessible at https://db.cngb.org/cnsa, and the Genome Sequence Archive (GSA)70 in National Genomics Data Center71, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences under accession number HRA009841, accessible at https://ngdc.cncb.ac.cn/gsa-human. All processed data can be accessed on Zenodo (https://zenodo.org/records/15400138)72. The bulk RNA-seq raw sequencing data have been deposited in GSA under accession number HRA011357. The reference genome used in this study is GRCh38 (https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000001405.26). The published datasets from nine external cohorts are available as follows: Cohort 1 data are in the original paper’s Supplementary Table 7 (ref. 24); Cohort 2 data are available in the database of Genotypes and Phenotypes (dbGaP) under accession number phs001404.v1.p1 (ref. 27); Cohort 3 data are on cBioPortal under accession number gbc_mskcc_2022 (ref. 28). Cohorts 4–8 data are deposited in the European Genome–Phenome Archive (EGA) under accession numbers EGAD00001004853, EGAD00001004854 and EGAD00001004855 (ref. 20); Gene Expression Omnibus (GEO) under accession numbers GSE201425 (ref. 64) and GSE126030 (ref. 65); GSA under BioProject ID PRJCA007744 (ref. 16); and an online data browser (http://cancer-pku.cn:3838/PanC_T)42. Cohort 9 data are available from the original study’s corresponding author upon request66. Source data are provided with this paper.
Code availability
The software and analysis code for reproducing the results are available on GitHub (https://github.com/JulieBaker1/AIEPI and https://github.com/JulieBaker1/GBC_analysis)73,74.
References
Roa, J. C. et al. Gallbladder cancer. Nat. Rev. Dis. Prim. 8, 69 (2022).
Valle, J. W., Kelley, R. K., Nervi, B., Oh, D.-Y. & Zhu, A. X. Biliary tract cancer. Lancet 397, 428–444 (2021).
de Visser, K. E. & Joyce, J. A. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 41, 374–403 (2023).
Patras, L., Shaashua, L., Matei, I. & Lyden, D. Immune determinants of the pre-metastatic niche. Cancer Cell 41, 546–572 (2023).
Lavin, Y. et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765 (2017).
Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845 (2019).
Izar, B. et al. A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 26, 1271–1279 (2020).
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459 (2020).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624 (2017).
Sun, Y. et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 184, 404–421 (2021).
Wang, R. et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat. Med. 27, 141–151 (2021).
Wu, S. Z. et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet 53, 1334–1347 (2021).
Becker, W. R. et al. Single-cell analyses define a continuum of cell state and composition changes in the malignant transformation of polyps to colorectal cancer. Nat. Genet. 54, 985–995 (2022).
Liu, Y. et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 40, 424–437 (2022).
Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612, 141–147 (2022).
Yeo, A. T. et al. Single-cell RNA sequencing reveals evolution of immune landscape during glioblastoma progression. Nat. Immunol. 23, 971–984 (2022).
Wang, R. et al. Evolution of immune and stromal cell states and ecotypes during gastric adenocarcinoma progression. Cancer Cell 41, 1407–1426 (2023).
Zhang, Y. et al. Single-cell RNA-seq reveals transcriptional landscape and intratumor heterogenicity in gallbladder cancer liver metastasis microenvironment. Ann. Transl. Med. 9, 889–889 (2021).
Wang, X. et al. Single-cell dissection of remodeled inflammatory ecosystem in primary and metastatic gallbladder carcinoma. Cell Discov. 8, 101 (2022).
Chen, P. et al. Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single‐cell RNA sequencing. Clin. Transl. Med. 11, e462 (2021).
Zhang, Y. et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J. Hepatol. 75, 1128–1141 (2021).
Li, M. et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat. Genet. 46, 872–876 (2014).
Li, M. et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut 68, 1024–1033 (2019).
He, H. et al. Comprehensive single-cell analysis deciphered microenvironmental dynamics and immune regulator olfactomedin 4 in pathogenesis of gallbladder cancer. Gut 73, 1529–1542 (2024).
Barkley, D. et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 54, 1192–1201 (2022).
Nepal, C. et al. Integrative molecular characterisation of gallbladder cancer reveals micro-environment-associated subtypes. J. Hepatol. 74, 1132–1144 (2021).
Giraldo, N. A. et al. Comprehensive molecular characterization of gallbladder carcinoma and potential targets for intervention. Clin. Cancer Res. 28, 5359–5367 (2022).
Makohon-Moore, A. P. et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 49, 358–366 (2017).
Spain, L. et al. Late-stage metastatic melanoma emerges through a diversity of evolutionary pathways. Cancer Discov. 13, 1364–1385 (2023).
Kulman, E., Wintersinger, J. & Morris, Q. Reconstructing cancer phylogenies using Pairtree, a clone tree reconstruction algorithm. STAR Protoc. 3, 101706 (2022).
Kaufmann, T. L. et al. MEDICC2: whole-genome doubling aware copy-number phylogenies for cancer evolution. Genome Biol. 23, 241 (2022).
Minesaki, A., Kai, K., Kuratomi, Y. & Aishima, S. Infiltration of CD1a-positive dendritic cells in advanced laryngeal cancer correlates with unfavorable outcomes post-laryngectomy. BMC Cancer 21, 973 (2021).
Wu, L. et al. Natural coevolution of tumor and immunoenvironment in glioblastoma. Cancer Discov. 12, 2820–2837 (2022).
van der Leun, A. M., Thommen, D. S. & Schumacher, T. N. CD8+T cell states in human cancer: insights from single-cell analysis. Nat. Rev. Cancer 20, 218–232 (2020).
Payen, V. L. et al. Single-cell RNA sequencing of human liver reveals hepatic stellate cell heterogeneity. JHEP Rep. 3, 100278 (2021).
O-charoenrat, P. et al. Epidermal growth factor-like ligands differentially up-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res. 60, 1121–1128 (2000).
Castillo, J. et al. Amphiregulin contributes to the transformed phenotype of human hepatocellular carcinoma cells. Cancer Res. 66, 6129–6138 (2006).
Wang, X. et al. EGFR is a master switch between immunosuppressive and immunoactive tumor microenvironment in inflammatory breast cancer. Sci. Adv. 8, eabn7983 (2022).
Santos, I. et al. CXCL5-mediated recruitment of neutrophils into the peritoneal cavity of Gdf15-deficient mice protects against abdominal sepsis. Proc. Natl Acad. Sci. USA 117, 12281–12287 (2020).
Chu, Y. et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 29, 1550–1562 (2023).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Song, X. et al. Overview of current targeted therapy in gallbladder cancer. Sig. Transduct. Target Ther. 5, 230 (2020).
Guo, W. et al. scCancer: a package for automated processing of single-cell RNA-seq data in cancer. Brief. Bioinform. 22, bbaa127 (2021).
Bais, A. S. & Kostka, D. scds: Computational annotation of doublets in single-cell RNA sequencing data. Bioinformatics 36, 1150–1158 (2020).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809 (2021).
Tang, F. et al. A pan-cancer single-cell panorama of human natural killer cells. Cell 186, 4235–4251 (2023).
Yang, Y. et al. Pan-cancer single-cell dissection reveals phenotypically distinct B cell subtypes. Cell 187, 4790–4811 (2024).
Wu, Y. et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell 187, 1422–1439 (2024).
Chen, J. et al. Transformer for one stop interpretable cell type annotation. Nat. Commun. 14, 223 (2023).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Wu, Y. et al. Highly Regional Genes: graph-based gene selection for single-cell RNA-seq data. J. Genet. Genomics 49, 891–899 (2022).
Schubach, M., Maass, T., Nazaretyan, L., Röner, S. & Kircher, M. CADD v1.7: using protein language models, regulatory CNNs and other nucleotide-level scores to improve genome-wide variant predictions. Nucleic Acids Res. 52, D1143–D1154 (2024).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Chen, J. et al. A highly heterogeneous mutational pattern in POEMS syndrome. Leukemia 35, 1100–1107 (2021).
Bai, J. et al. Whole genome sequencing of skull-base chordoma reveals genomic alterations associated with recurrence and chordoma-specific survival. Nat. Commun. 12, 757 (2021).
Alig, S. K. et al. Distinct Hodgkin lymphoma subtypes defined by noninvasive genomic profiling. Nature 625, 778–787 (2024).
Wintersinger, J. A. et al. Reconstructing complex cancer evolutionary histories from multiple bulk DNA samples using Pairtree. Blood Cancer Discov. 3, 208–219 (2022).
Shi, X. et al. Single-cell atlas of diverse immune populations in the advanced biliary tract cancer microenvironment. NPJ Precis. Onc. 6, 58 (2022).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 10, 4706 (2019).
Pandey, A. et al. Integrated genomic analysis reveals mutated ELF3 as a potential gallbladder cancer vaccine candidate. Nat. Commun. 11, 4225 (2020).
Lu, Y. et al. Occludin is a target of Src kinase and promotes lipid secretion by binding to BTN1a1 and XOR. PLoS Biol. 20, e3001518 (2022).
Zhou, T. et al. Occludin protects secretory cells from ER stress by facilitating SNARE-dependent apical protein exocytosis. Proc. Natl Acad. Sci. USA 117, 4758–4769 (2020).
Guo, X. et al. CNSA: a data repository for archiving omics data. Database (Oxf.) 2020, baaa055 (2020).
Chen, T. et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics 19, 578–583 (2021).
CNCB-NGDC Members and Partners. Database resources of the National Genomics Data Center, China National Center for Bioinformation in 2024. Nucleic Acids Res. 52, D18–D32 (2024).
Zhou, T., Wu, Y., Li, S., Gu, J. & Chen, L. Datasets for: multi-omic analysis of gallbladder cancer identifies distinct tumor microenvironments associated with disease progression (v 1.0.0). Zenodo https://doi.org/10.5281/zenodo.15400138 (2025).
Zhou, T., Wu, Y., Li, S., Gu, J. & Chen, L. AI-EPI: atlas-level integrated epithelial program identification (v 1.0.0). Zenodo https://doi.org/10.5281/zenodo.15406569 (2025).
Zhou, T., Wu, Y., Li, S., Gu, J. & Chen, L. Analysis code for: multi-omic analysis of gallbladder cancer identifies distinct tumor microenvironments associated with disease progression (v 1.0.0). Zenodo https://doi.org/10.5281/zenodo.15406874 (2025).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 82588201 to H.W., no. 82425038 to L.C., no. U21A20376 to L.C., no. 82273277 to X.Q., no. 82421005 to L.C., no. 82372872 to S.Y. and no. 62133006 to J.G.), National Key R&D Program of China (no. 2022YFC3400903 to L.C. and no. 2023YFC2507500 to L.C.), the National Science Foundation of Shanghai (no. 22140901000 to L.C.) and Shanghai Municipal Science and Technology Major Project.
Author information
Authors and Affiliations
Contributions
T.Z., Y.W., S.L., G.M. and W.G. analyzed sequencing data. T.Z., S.L., X.Q., E.L., X.Y., Y.Z., J.H., S.S. and S.Y. conducted experiments. Z.X., X.S. and X.W. collected clinical samples for sequencing and organized clinical information. K.W. collected clinical samples for experiments. T.Z., Y.W. and S.L. organized the figures. T.Z. wrote the manuscript. L.C., J.G., H.W., J.F. and X.J. designed the research, supervised the study, guided the discussion and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cell type definition and patient cohort information, related to Fig. 1.
a, UMAP plots showing the expression of classic marker genes of cell types, including B cells (CD79A+), dendritic cells (CLEC10A+), endothelial cells (VWF+), epithelial cells (EPCAM+), mesenchymal cells (PDGFRB+), mast cells (CPA3+), monocytes & macrophages (CD14+, FCGR3A+, and CD68+), neutrophils (FCGR3B+ and CSF3R+), NK & T cells (CD3D+ and NKG7+), plasma cells (MZB1+), and neuroendocrine tumor cells (NET cells) (INSM1+). b, Stacked barplots showing the distribution of non-tumor cell types in each sample, with detailed clinical attributes. c, Pie charts depicting the composition of different gallbladder diseases in our cohort. d, Pie charts depicting the composition of different sites for Adeno, Adeno Squa, and Neuro respectively. e-g, Pie charts depicting the composition of risk factors for GBC, including age (e), polyp & gallstone (f), and sex (g). F, female; M, male. XGC, xanthogranulomatous cholecystitis; CC, chronic cholecystitis; LG, low-grade neoplasia; HG, high-grade neoplasia; Adeno, adenocarcinomas; Adeno Squa, adenosquamous carcinomas; Neuro, neuroendocrine neoplasms; Undiff, undifferentiated carcinomas; Squa, squamous carcinoma; GB, gallbladder; Pri, primary tumors; PO, polyps; LI, liver invasive lesions; LN, lymph node metastases; LM, liver metastases; OM, omentum metastases.
Extended Data Fig. 2 Analysis of cell types and definition of subtypes, related to Fig. 1.
a, Kaplan-Meier plots illustrating the overall survival (OS) of patients grouped by cell percentages across cell types. The log-rank test was used. b, Ro/e indicating tissue site preference of cell types in gallbladder adenocarcinoma patients. c, Boxplots showing tissue site preference of B, dendritic, mast, and mesenchymal cells. n for Pri, PO, LN, LI, LM, and OM groups are 75, 1, 11, 10, 5, and 2. The Benjamini-Hochberg-adjusted two-sided Wilcoxon test was used. d, Boxplots showing sex preference of plasma and dendritic cells. n for male and female groups are 24 and 51. The two-sided Wilcoxon test was used. e-i, UMAP plots showing the expression of canonical marker genes of cell subtypes, including CD8+ T cell subtypes (e), CD4+ T cell subtypes (f), DC subtypes (g), endothelial cell subtypes (h), mesenchymal cell subtypes (i). Different histological classifications (XGC, CC, LG, etc.) combined with different tissue sites (Pri, PO, LI, etc.) form more refined groups. For example, Adeno_Pri represents primary tumors of adenocarcinoma patients. For boxplots in c and d, the center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. n represents biologically independent samples.
Extended Data Fig. 3 Epithelial classification and genomic landscape of GBC, related to Fig. 1.
a, The gene expression UMAP plots showing group identifications in epithelial cells, colored by histological types, sites, as well as gallstone and polyp information. b, The copy number variation (cnv) UMAP plots showing epithelial cnv_leiden, cnv_score, and cnv_status. The cnv_status was also labeled in the gene expression UMAP plot. c, Significantly mutated genes in GBC. Detailed clinical features were annotated. The top barplot shows the somatic mutation number. The right barplot shows the mutation frequency. The left bar plot shows the significance: -Log10 (P) values are shown in light gray, and -Log (q) values are shown in dark gray. The red vertical line represents a P value of 0.05. The permutation test was used. d-e, Boxplots showing the enrichment of TMB across TNM stages (d) and tissue sites (e). According to the TNM stages, n are sequentially 3, 8, 6, 16, 18, and 35. n for Pri, PO, LN, LI, LM, and OM groups are 86, 2, 12, 11, 6, and 2. The one-way ANOVA test was used. TMB, tumor mutational burden. f, Comparison of mutation frequencies among 22 genes that occur in more than 5% of samples in Cohort 3. For boxplots in d and e, the center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. n represents biologically independent samples.
Extended Data Fig. 4 Paired comparisons between primary tumors and metastases, related to Fig. 1.
a, CNV landscape of paired samples in GBC patients. b, Boxplot of the Jaccard similarity coefficient of paired samples (n = 20). The center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. c, Venn diagram of the shared mutations among multi-lesions of a patient. d, Clone-level and copy-number-level phylogenies inferred for the 4 patients. n represents biologically independent samples.
Extended Data Fig. 5 Macrophage changes during disease progression, related to Fig. 2.
a, The expression of differentially expressed genes (DEGs) across macrophage subtypes. b, The scores of functional signatures of tumor-associated macrophage (TAM) subsets across macrophage subtypes. c, Heatmap showing distinct disease prevalence estimated by Ro/e. The top barplot shows cell composition, and the right barplot shows sample composition across distinct diseases. d-f, Boxplots showing the proportions of macrophage subtypes between Non-tumor (containing CC_GB, XGC_GB, LG_GB, and HG_GB, n = 16) and Adeno_Pri (n = 75) (d), paired NAT (normal tissues adjacent to the tumors, n = 21) and Adeno_Pri (n = 21) (e), and P (n = 20) and P_Mets (n = 55) (f). g, Differentially enriched pathways of M_C1_S100A8 between Non-tumor and Adeno_Pri. The Benjamini-Hochberg-adjusted one-sided hypergeometric test was used. h, Heatmap showing distinct tissue site prevalence estimated by Ro/e across macrophage subtypes. i, Pearson correlation between the percentages of M_C1_S100A8 in Adeno_Pri and Adeno_LN, with 95% confidence interval shown. The two-sided t-test was used. j, Volcano plots showing DEGs in P_Mets versus P across macrophage subtypes. Up-regulated chemokines were displayed. The Benjamini-Hochberg-adjusted two-sided Wilcoxon test was used. For d, e, and f, the two-sided Wilcoxon test was used. For boxplots in d, e, and f, the center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. n represents biologically independent samples.
Extended Data Fig. 6 Characterization of Lymphoid subtypes in Adeno_Pri, related to Fig. 4.
a-d, Bubble plots showing the expression of DEGs across subtypes for each cell type, including CD8+ T cells (a), CD4+ T cells (b), NK cells (c), and B cells (d). Heatmaps show the prevalence of distinct diseases estimated by Ro/e. The right barplots show sample composition. The bottom barplots show cellular composition. e, Boxplots compare the proportions of lymphoid subtypes between Non-tumor (n = 16) and Adeno_Pri (n = 75). The center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. f, The scores of functional signatures and the expression of immune checkpoint genes across lymphoid subtypes. g, Kaplan-Meier plots illustrating OS of patients grouped by the percentages of lymphoid subtypes. The log-rank test was used. h, The comparison of the score of the immune checkpoint gene set in CD4T_C1_FOXP3_TREG between Non-tumor and Adeno_Pri. The inner boxplot displays the median (center dot), the interquartile range (box limits), and whiskers that extend to 1.5 times the interquartile range from the quartiles. i, DEGs of NK_C3_CAPG versus other NK cells. For e, h, and i, the two-sided Wilcoxon test was used. n represents biologically independent samples.
Extended Data Fig. 7 Characterization of Lymphoid subtypes in P_Mets, related to Fig. 4.
a, The proportions of lymphoid subtypes between P (n = 20) and P_Mets (n = 55). The center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. b, Scores of functional gene sets in CD4T_C2_CCR7_TN between P (n = 20) and P_Mets (n = 55). c, DEGs of CD4T_C2_CCR7_TN between P and P_Mets. d, AREG expression in CD4T_C2_CCR7_TN in Adeno_Pri. e, The dynamic change of AREG expression in CD4T_C2_CCR7_TN across TNM stages. f, Scatter plots illustrating the proportions of subtypes per patient. Lines represent the results of linear regression fitting (two-sided t-test). g, Pearson correlation across the percentages of CD4+ T cell subtypes. h, The scores of different functional gene sets in CD4T_C2_CCR7_TN across TNM stages. The right line plots show the dynamic changes in functions of CD4T_C2_CCR7_TN along with TNM stages. i, DEGs of CD4T_C2_CCR7_TN between Adeno_Pri and Adeno_LN. j, DEGs and enriched pathways of naïve CD4+ T cells between Normal_LN and Adeno_LN. k, AREG expression of naïve CD4+ T cells in another GBC cohort. l, The UMAP of CD4+ T cell subclusters in an external HCC cohort. Sample information of the TN cluster is denoted in the lower right box. m, AREG expression of CD4+ T cell subtypes in a pan-cancer dataset. The bottom panel shows the expression of AREG and classical naïve T cell markers. n, Differentially enriched pathways of CD4T_C2_CCR7_TN among LG, HG, XGC, and Adeno_Pri. For a and b, the two-sided Wilcoxon test was used. For c and i, the Benjamini-Hochberg-adjusted two-sided Wilcoxon test was used. For j and n, the Benjamini-Hochberg-adjusted one-sided hypergeometric test was used. For violin plots in b and m, the inner boxplot displays the median (center line or dot), the interquartile range (box limits), and whiskers that extend to 1.5 times the interquartile range from the quartiles. n represents biologically independent samples.
Extended Data Fig. 8 The pro-angiogenic role of stromal cell subtypes, related to Fig. 5.
a, DEG expression across mesenchymal subtypes. b, Heatmap showing distinct disease prevalence estimated by Ro/e. The top barplot shows cell composition and the right barplot shows sample composition across distinct diseases. c-e, Boxplots compare the proportions of mesenchymal subtypes between Non-tumor (n = 16) and Adeno_Pri (n = 75) (c), paired NAT (n = 21) and Adeno_Pri (n = 21) (d), and P (n = 20) and P_Mets (n = 55) (e). f, Boxplots showing tissue site preference of F_C7_COL4A2. g-h, The DEGs (g) and enriched pathways (h) of F_C1_CFD_iCAF between P and P_Mets. i, VEGFB expression across mesenchymal subtypes. j, Kaplan-Meier plots illustrating OS of patients grouped by the percentage of F_C1_CFD_iCAF. k-l, The DEGs (k) and enriched pathways (l) of endothelial cell subtypes. m, Heatmap showing distinct disease prevalence estimated by Ro/e. The top barplot shows cell composition and the right barplot shows sample composition across distinct diseases. n-p, Boxplots compare the proportions of endothelial cell subtypes between Non-tumor (n = 16) and Adeno_Pri (n = 75) (n), paired NAT (n = 21) and Adeno_Pri (n = 21) (o), and P (n = 20) and P_Mets (n = 55) (p). q, Pearson correlation between the percentages of EC_C2_CXCR4_Tip and EC_C0_ACKR1_Vein (left panel) or EC_C3_GJA5_Artery (right panel), with 95% confidence interval shown. The two-sided t-test was used. r, FLT1 expression across classic endothelial cell subtypes. s, Kaplan-Meier plots illustrating OS of patients grouped by the percentage of EC_C3_GJA5_Artery. For c, d, e, n, o, and p, the two-sided Wilcoxon test was used. For f and g, the Benjamini-Hochberg-adjusted two-sided Wilcoxon test was used. For h and l, the Benjamini-Hochberg-adjusted one-sided hypergeometric test was used. For j and s, the log-rank test was used. For boxplots in c, d, e, n, o, and p, the center line represents the median, box edges show quartiles, whiskers extend 1.5 times the interquartile range, and points beyond are individual outliers. n represents biologically independent samples.
Extended Data Fig. 9 Stratification of adenocarcinoma microenvironments, related to Fig. 5.
a, The dotplot showing the significance of enrichment of cell subtypes in each somatic mutation group (The number of samples in each group needs to be greater than 3). The size of the dots represents the P value, with gray dots indicating non-significance, red dots indicating a significant increase in cell proportion, and green dots indicating a significant decrease. The two-sided Wilcoxon test was used. b, The dotplot showing the significance of enrichment of cell subtypes in each MI group. The size of the dots represents the P value, with gray dots indicating non-significance, red dots indicating a significant increase in cell proportion, and green dots indicating a significant decrease. Colors indicate different types of MI. The two-sided Wilcoxon test was used. c, PCA visualization of samples. Colors represent different MI types. d, The heatmap showing the accuracy between actual and predicted results. e, Somatic mutations across 5 MIs. The right values show the mutation frequency.
Extended Data Fig. 10 AREG-CXCL5 recruits neutrophils and resists therapy, related to Fig. 7.
a, Relative proliferative rates of GBC-SD cells under the treatment with AREG or not as detected by CCK8 assay. b, Representative images of migration and invasion assays under different concentrations of AREG treatment using GBC-SD cells. Scale bar: 100 μm. c, mRNA and protein expression of CXCL5 as detected by qPCR and ELISA respectively under different conditions as denoted. Both assays were conducted on SGC996 cells. For qPCR, values were normalized against actin expression. d, mRNA and protein expression of CXCL5 as detected by qPCR and ELISA respectively under different conditions as denoted. Both assays were conducted on organoids. For qPCR, values were normalized against actin expression. The two-sided paired t-test was used. e, Protein expression of EGR1, pERK1/2, and total ERK1/2 as detected by western blotting under the conditions as denoted using SGC996 cells. Internal control was β-Tubulin. f, Protein expression of EGR1, pERK1/2, and total ERK1/2 as detected by western blotting under the conditions as denoted using organoids. Internal control was β-Tubulin. g, The ratio of liver weight to body weight for each mouse across the four groups. h, Neutrophil infiltration as detected by IHC with CD66b staining in tumor sections across groups. CI, control SGC996 cells and IgG; CP, control SGC996 cells and anti-PD-L1; AI, SGC996 cells treated by AREG and IgG; AP, SGC996 cells treated by AREG and anti-PD-L1. Scale bar: 50 μm. For a, b, c, and g, the two-sided unpaired t-test was used. Data are presented as mean values ± SD. n represents biologically independent samples and all experiments were performed at least three times, unless specified otherwise.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Supplementary Notes 1–11, Supplementary Discussion and Supplementary References.
Source data
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data and unprocessed western blots.
Source Data Fig. 7
Statistical Source Data and unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data and unprocessed western blots.
Source Data Extended Data Fig. 10
Statistical Source Data and unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, T., Wu, Y., Li, S. et al. Multi-omic analysis of gallbladder cancer identifies distinct tumor microenvironments associated with disease progression. Nat Genet 57, 1935–1949 (2025). https://doi.org/10.1038/s41588-025-02236-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41588-025-02236-9
This article is cited by
-
Spatial and temporal dynamics in progressive gallbladder cancer
Nature Genetics (2025)