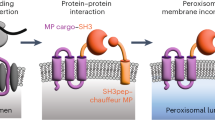
Abstract
Repurposing an organelle for specialized metabolism provides an avenue for fermentable, unicellular organisms such as Saccharomyces cerevisiae to mimic compartmentalization of metabolic pathways within different plant tissues. Peroxisomes are attractive organelles for repurposing as they are not required for yeast viability when grown on glucose and can efficiently compartmentalize heterologous enzymes to enable physical separation of cytosolic native metabolism and peroxisomal engineered metabolism. However, when not required, peroxisomes are repressed, leading to low functional capacities for heterologous proteins. Here we engineer peroxisomes with enhanced functional capacities, with the goal of compartmentalizing up to eight metabolic enzymes to enhance titers. We implement a machine learning pipeline that allows the identification of factors to overexpress, culminating in a 137% increase in peroxisome functional capacity compared to a wild-type strain. Improved pathway compartmentalization enables an 80% increase in the biosynthesis titers of the monoterpene geraniol, up to 9.5 g L−1.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Plasmids generated in this study were deposited to Addgene (plasmids 218587–218627) and yeast strains are available upon request. Data files, including raw data underlying figures were uploaded to figshare repository (https://doi.org/10.6084/m9.figshare.26156098.v1) (ref. 59). The data that support the findings of this study are available within the main text and the Supplementary Information. Data are also available from the corresponding authors upon request.
Code availability
ML data analysis codes are available from GitHub (https://github.com/CCCofficial/ML_Pipeline_Yeast_Peroxisome) and Zenodo (https://doi.org/10.5281/zenodo.13334581) (ref. 60).
Change history
08 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41589-024-01784-1
References
Heinig, U., Gutensohn, M., Dudareva, N. & Aharoni, A. The challenges of cellular compartmentalization in plant metabolic engineering. Curr. Opin. Biotechnol. 24, 239–246 (2013).
Lazarow, P. B. Rat liver peroxisomes catalyze the β oxidation of fatty acids. J. Biol. Chem. 253, 1522–1528 (1978).
Keller, G. A., Gould, S., Deluca, M. & Subramani, S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc. Natl Acad. Sci. USA 84, 3264–3268 (1987).
Hayashi, M. et al. Functional transformation of plant peroxisomes. Cell Biochem. Biophys. 32, 295–304 (2000).
Fung, K. & Clayton, C. Recognition of a peroxisomal tripeptide entry signal by the glycosomes of Trypanosoma brucei. Mol. Biochem. Parasitol. 45, 261–264 (1991).
Kiel, J. A. K. W., Hilbrands, R. E., Bovenberg, R. A. L. & Veenhuis, M. Isolation of Penicillium chrysogenum PEX1 and PEX6 encoding AAA proteins involved in peroxisome biogenesis. Appl. Microbiol. Biotechnol. 54, 238–242 (2000).
Purdue, P. E. & Lazarow, P. B.Peroxisome biogenesis. Annu. Rev. Cell Dev. Biol. 17, 701–752 (2001).
DeLoache, W. C., Russ, Z. N. & Dueber, J. E. Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways. Nat. Commun. 7, 11152 (2016).
Dusséaux, S., Wajn, W. T., Liu, Y., Ignea, C. & Kampranis, S. C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl Acad. Sci. USA 117, 31789–31799 (2020).
Grewal, P. S., Samson, J. A., Baker, J. J., Choi, B. & Dueber, J. E. Peroxisome compartmentalization of a toxic enzyme improves alkaloid production. Nat. Chem. Biol. 17, 96–103 (2021).
Choi, B. H., Kang, H. J., Kim, S. C. & Lee, P. C. Organelle engineering in yeast: enhanced production of protopanaxadiol through manipulation of peroxisome proliferation in Saccharomyces cerevisiae. Microorganisms 10, 650 (2022).
Sheng, J., Stevens, J. & Feng, X. Pathway compartmentalization in peroxisome of Saccharomyces cerevisiae to produce versatile medium chain fatty alcohols. Sci. Rep. 6, 26884 (2016).
Davies, M. E., Tsyplenkov, D. & Martin, V. J. J. Engineering yeast for de novo synthesis of the insect repellent nepetalactone. ACS Synth. Biol. 10, 2896–2903 (2021).
Gerke, J. et al. Production of the fragrance geraniol in peroxisomes of a product-tolerant baker’s yeast. Front. Bioeng. Biotechnol. 8, 582052 (2020).
Erdmann, R. & Blobel, G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 128, 509–523 (1995).
Deb, R. & Nagotu, S.The nexus between peroxisome abundance and chronological ageing in Saccharomyces cerevisiae. Biogerontology 24, 81–97 (2022).
Huber, A., Koch, J., Kragler, F., Brocard, C. & Hartig, A. A subtle interplay between three Pex11 proteins shapes de novo formation and fission of peroxisomes. Traffic 13, 157–167 (2012).
Krikken, A. M., Veenhuis, M. & van der Klei, I. J. Hansenula polymorpha pex11 cells are affected in peroxisome retention. FEBS J. 276, 1429–1439 (2009).
Greenhalgh, J. C., Fahlberg, S. A., Pfleger, B. F. & Romero, P. A. Machine learning-guided acyl-ACP reductase engineering for improved in vivo fatty alcohol production. Nat. Commun. 12, 5825 (2021).
Mukherjee, M., Blair, R. H. & Wang, Z. Q. Machine-learning guided elucidation of contribution of individual steps in the mevalonate pathway and construction of a yeast platform strain for terpenoid production. Metab. Eng. 74, 139–149 (2022).
Wang, S. et al. Massive computational acceleration by using neural networks to emulate mechanism-based biological models. Nat. Commun. 10, 4354 (2019).
Radivojević, T., Costello, Z., Workman, K. & Garcia Martin, H. A machine learning automated recommendation tool for synthetic biology. Nat. Commun. 11, 4879 (2020).
Daniels, K. G. et al. Decoding CAR T cell phenotype using combinatorial signaling motif libraries and machine learning. Science 378, 1194–1200 (2022).
Luo, N., Wang, S., Lu, J., Ouyang, X. & You, L. Collective colony growth is optimized by branching pattern formation in Pseudomonas aeruginosa. Mol. Syst. Biol. 17, e10089 (2021).
Wu, Z., Kan, S. B. J., Lewis, R. D., Wittmann, B. J. & Arnold, F. H. Machine learning-assisted directed protein evolution with combinatorial libraries. Proc. Natl Acad. Sci. USA 116, 8852–8858 (2019).
Chen, W. & Viljoen, A. M. Geraniol—a review of a commercially important fragrance material. S. Afr. J. Bot. 76, 643–651 (2010).
Rubat, S. et al. Increasing the intracellular isoprenoid pool in Saccharomyces cerevisiae by structural fine-tuning of a bifunctional farnesyl diphosphate synthase. FEMS Yeast Res. 17, fox032 (2017).
Zhao, J. et al. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb. Cell Fact. 16, 17 (2017).
Ignea, C., Pontini, M., Maffei, M. E., Makris, A. M. & Kampranis, S. C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 3, 298–306 (2014).
Lee, M. E., DeLoache, W. C., Cervantes, B. & Dueber, J. E. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth. Biol. 4, 975–986 (2015).
van Roermund, C. W. T., Tabak, H. F., van den Berg, M., Wanders, R. J. A. & Hettema, E. H. Pex11p plays a primary role in medium-chain fatty acid oxidation, a process that affects peroxisome number and size in Saccharomyces cerevisiae. J. Cell Biol. 150, 489–498 (2000).
Tam, Y. Y. C. et al. Pex11-related proteins in peroxisome dynamics: a role for the novel peroxin Pex27p in controlling peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 4089–4102 (2003).
Lin, P. et al. Direct utilization of peroxisomal acetyl-CoA for the synthesis of polyketide compounds in Saccharomyces cerevisiae. ACS Synth. Biol. 12, 1599–1607 (2023).
Yofe, I. et al. Pex35 is a regulator of peroxisome abundance. J. Cell Sci. 130, 791–804 (2017).
Rottensteiner, H., Stein, K., Sonnenhol, E. & Erdmann, R. Conserved function of Pex11p and the novel Pex25p and Pex27p in peroxisome biogenesis. Mol. Biol. Cell 14, 4316–4328 (2003).
Tower, R. J., Fagarasanu, A., Aitchison, J. D. & Rachubinski, R. A. The peroxin Pex34p functions with the Pex11 family of peroxisomal divisional proteins to regulate the peroxisome population in yeast. Mol. Biol. Cell 22, 1727–1738 (2011).
Hoepfner, D., van den Berg, M., Philippsen, P., Tabak, H. F. & Hettema, E. H. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155, 979–990 (2001).
Wróblewska, J. P. & van der Klei, I. J. Peroxisome maintenance depends on de novo peroxisome formation in yeast mutants defective in peroxisome fission and inheritance. Int. J. Mol. Sci. 20, 4023 (2019).
Yuan, W., Veenhuis, M. & van der Klei, I. J. The birth of yeast peroxisomes. Biochim. Biophys. Acta 1863, 902–910 (2016).
Wittmann, B. J., Yue, Y. & Arnold, F. H. Informed training set design enables efficient machine learning-assisted directed protein evolution. Cell Syst. 12, 1026–1045 (2021).
Pandi, A. et al. A versatile active learning workflow for optimization of genetic and metabolic networks. Nat. Commun. 13, 3876 (2022).
Zhang, J. et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism. Nat. Commun. 11, 4880 (2020).
Peng, B. et al. A squalene synthase protein degradation method for improved sesquiterpene production in Saccharomyces cerevisiae. Metab. Eng. 39, 209–219 (2017).
Zhao, J., Bao, X., Li, C., Shen, Y. & Hou, J. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 100, 4561–4571 (2016).
Osterberg, M., Kim, H., Warringer, J. & von Heijne, G. Phenotypic effects of membrane protein overexpression in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 103, 11148–11153 (2006).
Pedregosa, F. et al. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Galton, F. Regression towards mediocrity in hereditary stature. J. R. Anthropol. Inst. 15, 246–263 (1886).
Cover, T. & Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 13, 21–27 (1967).
Ho, T. K. Random decision forests. In Proc. 3rd International Conference on Document Analysis and Recognition (eds Kavanaugh, M. & Storms, P.) (IEEE, 1995).
Vapnik, V. N. The support vector method. In Proc. Artificial Neural Networks—ICANN’97 (eds Gerstner, W. et al.) (Springer, 1997).
Friedman, J. H.Greedy function approximation: a gradient boosting machine. Ann. Statist. 29, 1189–1232 (2001).
O’Shea, K. & Nash, R. An introduction to convolutional neural networks. Preprint at https://arXiv.org/abs/1511.08458 (2015).
Lecun, Y., Bottou, L., Bengio, Y. & Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 86, 2278–2324 (1998).
Hochreiter, S. & Schmidhuber, J. Long short-term memory. Neural Comput. 9, 1735–1780 (1997).
Géron, A. Hands-On Machine Learning with Scikit-Learn, Keras, and TensorFlow: Concepts, Tools, and Techniques to Build Intelligent Systems (O’Reilly, 2019).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://arXiv.org/abs/1802.03426 (2020).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Tieleman, T. & Hinton, G. in Neural Networks for Machine Learning Vol. 4, 26–31 (COURSERA, 2012).
Baker, J. Engineering yeast peroxisomes to be high capacity for production of geraniol. figshare https://doi.org/10.6084/m9.figshare.26156098.v1 (2024).
Shi, J., Wang, S. & Capponi, S. CCCofficial/ML_Pipeline_Yeast_Peroxisome. Zenodo https://doi.org/10.5281/zenodo.13334581 (2024).
Acknowledgements
This work was funded by the Center for Cellular Construction, an NSF Science and Technology Center (grant number: DBI-1548297). This grant funded all authors.
Author information
Authors and Affiliations
Contributions
J.J.B. and J.E.D. designed the research. J.J.B. performed the DNA cloning, transformations, microscopy, degron assay, tNCS assay, protease assay and geraniol experiments. E.M.M performed DNA cloning, transformations and geraniol experiments. S.B., S.C. and S.W. conceptualized the computational approach. J.S. and S.W. created and implemented the ML pipeline with supervision from S.C. Biological data were analyzed by J.J.B. and ML data were analyzed by J.S. under the supervision of S.W. J.E.D supervised the research. J.J.B., J.E.D., J.S. and S.C. wrote the paper and created the figures.
Corresponding authors
Ethics declarations
Competing interests
J.S., J.E.D., J.J.B., S.C. and S.W. are listed as inventors on a patent filed by IBM (application number: 18/657280; filed on May 7, 2024) titled ‘ML pipeline for efficient exploration of combinatorial space’. The patent covers the ML algorithm presented within. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Phil Gitman, Edoardo Saccenti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Log2 fold change in transcription of genes related to peroxisomes between a strain with engineered TFs (constitutively expressed and constitutively active version of Adr1, Oaf1, and Pip2) and WT.
From the GO: 0007031 term related to peroxisome organization, only the TFs that were constitutively expressed had more than a log2-fold change greater than 0.5. The remaining genes all fall within a log2 fold change of -0.36 and 0.41, indicating relatively small changes to transcription levels for genes involved in peroxisome organization. In comparison, other oleate-induction transcriptomics results report log2 fold changes greater than 5 for other genes, even for some membrane proteins. Thus, these TF-induced transcription changes are minimal compared to other studies. From the GO: 0005777 term related to peroxisomes, the genes with the highest increase in transcription were all beta-oxidation of fatty acid genes located in the peroxisome matrix and PEX11, involved in peroxisome fission. The proteins involved in beta-oxidation of fatty acids are unlikely to also control peroxisome morphology and proliferation. Pex11p has important roles controlling peroxisome fission and therefore morphology18. The genes most downregulated in the TFs compared to WT are: OPT2, CIT2, PNC1, PXP2, and MLS1. These genes encode proteins that localize to the peroxisome matrix but have no reported relationship to peroxisome proliferation, morphology, or oleate induction. Excluding these genes, the rest of the genes in this category, including all PEX genes (Supplementary Table 1) fall between a log2 fold of -0.506 and 0.530, indicating the rest of the genes in this category have relatively small changes in transcription. The GO: 000425 and 0030242 terms related to peroxisome pexophagy showed small downregulations in transcription for most of the genes. Overall, the data show small transcription increases to many genes and decreases in transcription to most pexophagy-related genes.
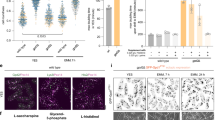
Extended Data Fig. 2 Use of a Tev protease assay shows an increased import rate of cargo into the enhanced capacity peroxisomes.
Scale bars in all microscopy images are 5 µm. (a) A TEV protease assay utilizes expression of RFP-TEV protease cleavage site-YFP-ePTS1 with varying expression levels of the TEV protease. At low expression of TEV protease, both RFP and YFP should be localized to the peroxisome as there is not enough TEV protease to cleave the fluorescent proteins before import to the peroxisome. At high expression of the TEV protease, there is competition between import rate and TEV cleavage. If TEV protease cleaves between the fluorescent proteins before import, only YFP will be imported to the peroxisome while RFP will remain cytosolic. At high TEV protease expression, WT capacity peroxisomes have a large quantity of RFP in the cytosol as visualized by the diffuse red fluorescence throughout the cell, meaning that TEV-catalyzed cleavage occurred before peroxisome import. In the enhanced peroxisome capacity strain, the bulk of the RFP is colocalized in the peroxisome, meaning faster import to the peroxisomes protects the fluorescent proteins from being cleaved from each other. Microscopy was performed two individual times with similar results. (b) Fluorescence microscopy of YFP-ePTS1 in WT, cells with the engineered TFs, and the ML predicted enhanced capacity peroxisome strain. The TFs gave singular, large peroxisomes compared to WT, while the enhanced capacity peroxisomes had many, bright peroxisomes that were not as large as the TFs, but led to an overall increase in functional capacity. Microscopy was performed two individual times with similar results.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Supplementary Tables 1 and 2
Supplementary Table 1: PEX gene transcription changes. Supplementary Table 2: Plasmid and strain information.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baker, J.J., Shi, J., Wang, S. et al. ML-enhanced peroxisome capacity enables compartmentalization of multienzyme pathway. Nat Chem Biol 21, 727–735 (2025). https://doi.org/10.1038/s41589-024-01759-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41589-024-01759-2
This article is cited by
-
Improved de novo production of prostaglandin F2α from glucose with engineered Yarrowia lipolytica strains
Communications Biology (2026)
-
Advances in the sustainable biosynthesis of valuable terpenoid flavor compounds and precursors in micro-organisms
Biotechnology for Biofuels and Bioproducts (2025)
-
De novo biosynthesis of taxifolin in yeast peroxisomes
Microbial Cell Factories (2025)
-
Engineering yeast peroxisome assembly enables the increased production of acetyl-CoA and its derived 5-deoxyflavonoids
Nature Communications (2025)
-
A peroxisomal membrane protein hub
Nature Chemical Biology (2025)