Abstract
Blocking pyrimidine de novo synthesis by inhibiting dihydroorotate dehydrogenase is used to treat autoimmunity and prevent expansion of rapidly dividing cell populations including activated T cells. Here we show memory T cell precursors are resistant to pyrimidine starvation. Although the treatment effectively blocked effector T cells, the number, function and transcriptional profile of memory T cells and their precursors were unaffected. This effect occurred in a narrow time window in the early T cell expansion phase when developing effector, but not memory precursor, T cells are vulnerable to pyrimidine starvation. This vulnerability stems from a higher proliferative rate of early effector T cells as well as lower pyrimidine synthesis capacity when compared with memory precursors. This differential sensitivity is a drug-targetable checkpoint that efficiently diminishes effector T cells without affecting the memory compartment. This cell fate checkpoint might therefore lead to new methods to safely manipulate effector T cell responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Sequencing data have been deposited in the GEO under the primary accession code GSE200360. Source data are provided with this paper. All other data supporting this study are available in the main article and Supplementary Information.
Code availability
The code used in the manuscript for processing and analysis of next-generation sequencing data can be found at https://github.com/gpdealmeida/zehn_nat_imm_2023/, https://hoohm.github.io/dropSeqPipe/ and https://gitlab.lrz.de/ImmunoPhysio/bulkSeqPipe/.
References
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Best, J. A. et al. Transcriptional insights into the CD8+ T cell response to infection and memory T cell formation. Nat. Immunol. 14, 404–412 (2013).
Lugli, E., Galletti, G., Boi, S. K. & Youngblood, B. A. Stem, effector and hybrid states of memory CD8+ T cells. Trends Immunol. 41, 17–28 (2020).
Pace, L. et al. The epigenetic control of stemness in CD8+ T cell fate commitment. Science 359, 177–186 (2018).
Geltink, R. I. K., Kyle, R. L. & Pearce, E. L. Unraveling the complex interplay between T cell metabolism and function. Annu Rev. Immunol. 36, 461–488 (2018).
Buchholz, V. R. et al. Disparate individual fates compose robust CD8+ T cell immunity. Science 340, 630–635 (2013).
Herndler-Brandstetter, D. et al. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity 48, 716–729 (2018).
Jameson, S. C. & Masopust, D. Understanding subset diversity in T cell memory. Immunity 48, 214–226 (2018).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Thome, J. J. et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828 (2014).
Urban, S. L. et al. Peripherally induced brain tissue-resident memory CD8+ T cells mediate protection against CNS infection. Nat. Immunol. 21, 938–949 (2020).
Arens, R. & Schoenberger, S. P. Plasticity in programming of effector and memory CD8 T cell formation. Immunol. Rev. 235, 190–205 (2010).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813 (2009).
Joshi, N. S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Kaech, S. M. et al. Selective expression of the interleukin-7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003).
Martin, M. D. & Badovinac, V. P. Defining memory CD8 T cell. Front Immunol. 9, 2692 (2018).
Hess Michelini, R., Doedens, A. L., Goldrath, A. W. & Hedrick, S. M. Differentiation of CD8 memory T cells depends on Foxo1. J. Exp. Med. 210, 1189–1200 (2013).
Jeannet, G. et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl Acad. Sci. USA 107, 9777–9782 (2010).
Kim, M. V., Ouyang, W., Liao, W., Zhang, M. Q. & Li, M. O. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity 39, 286–297 (2013).
Milner, J. J. et al. Heterogenous populations of tissue-resident CD8+ T cells are generated in response to infection and malignancy. Immunity 52, 808–824 (2020).
Zhao, D. M. et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J. Immunol. 184, 1191–1199 (2010).
Zhou, X. et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240 (2010).
Raczkowski, F. et al. The transcription factor Interferon regulatory factor 4 is required for the generation of protective effector CD8+ T cells. Proc. Natl Acad. Sci. USA 110, 15019–15024 (2013).
Starbeck-Miller, G. R., Xue, H. H. & Harty, J. T. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J. Exp. Med. 211, 105–120 (2014).
Sullivan, B. M., Juedes, A., Szabo, S. J., von Herrath, M. & Glimcher, L. H. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl Acad. Sci. USA 100, 15818–15823 (2003).
Xue, L., Chiang, L., He, B., Zhao, Y. Y. & Winoto, A. FoxM1, a forkhead transcription factor is a master cell cycle regulator for mouse mature T cells but not double positive thymocytes. PLoS ONE 5, e9229 (2010).
Yang, C. Y. et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12, 1221–1229 (2011).
Broen, J. C. A. & van Laar, J. M. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 16, 167–178 (2020).
Aly, L., Hemmer, B. & Korn, T. From leflunomide to teriflunomide: drug development and immunosuppressive oral drugs in the treatment of multiple sclerosis. Curr. Neuropharmacol. 15, 874–891 (2017).
Thoenes, G. H., Sitter, T., Langer, K. H., Bartlett, R. R. & Schleyerbach, R. Leflunomide (HWA 486) inhibits experimental autoimmune tubulointerstitial nephritis in rats. Int. J. Immunopharmacol. 11, 921–929 (1989).
Herrmann, M. L., Schleyerbach, R. & Kirschbaum, B. J. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 47, 273–289 (2000).
Cherwinski, H. M. et al. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J. Pharmacol. Exp. Ther. 275, 1043–1049 (1995).
Chong, A. S. et al. Leflunomide, a novel immunosuppressive agent. The mechanism of inhibition of T cell proliferation. Transplantation 55, 1361–1366 (1993).
Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Chan, V., Charles, B. G. & Tett, S. E. Population pharmacokinetics and association between A77 1726 plasma concentrations and disease activity measures following administration of leflunomide to people with rheumatoid arthritis. Br. J. Clin. Pharm. 60, 257–264 (2005).
Ahmed, R., Salmi, A., Butler, L. D., Chiller, J. M. & Oldstone, M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Moskophidis, D., Lechner, F., Pircher, H. & Zinkernagel, R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993).
Zehn, D. & Wherry, E. J. Immune memory and exhaustion: clinically relevant lessons from the LCMV model. Adv. Exp. Med Biol. 850, 137–152 (2015).
Kretschmer, L. et al. Differential expansion of T central memory precursor and effector subsets is regulated by division speed. Nat. Commun. 11, 113 (2020).
Quéméneur, L. et al. Restriction of de novo nucleotide biosynthesis interferes with clonal expansion and differentiation into effector and memory CD8 T cells. J. Immunol. 173, 4945–4952 (2004).
Araki, K. et al. Pathogenic virus-specific T cells cause disease during treatment with the calcineurin inhibitor FK506: implications for transplantation. J. Exp. Med. 207, 2355–2367 (2010).
Cannarile, M. A. et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 7, 1317–1325 (2006).
Intlekofer, A. M. et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 204, 2015–2021 (2007).
Kallies, A., Xin, A., Belz, G. T. & Nutt, S. L. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009).
Rutishauser, R. L. et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Prlic, M., Hernandez-Hoyos, G. & Bevan, M. J. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 203, 2135–2143 (2006).
Sarkar, S. et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205, 625–640 (2008).
Snell, L. M. et al. CD8+ T cell priming in established chronic viral infection preferentially directs differentiation of memory-like cells for sustained immunity. Immunity 49, 678–694 (2018).
Araki, K. et al. mTOR regulates memory CD8 T cell differentiation. Nature 460, 108–112 (2009).
Zhang, L. et al. Mammalian target of rapamycin complex 2 controls CD8 T cell memory differentiation in a Foxo1-dependent manner. Cell Rep. 14, 1206–1217 (2016).
Banerjee, A. et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185, 4988–4992 (2010).
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21–interleukin-10–STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Teixeiro, E. et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science 323, 502–505 (2009).
Badovinac, V. P., Porter, B. B. & Harty, J. T. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5, 809–817 (2004).
Bird, N. L. et al. Oseltamivir prophylaxis reduces inflammation and facilitates establishment of cross-strain protective T cell memory to influenza viruses. PLoS ONE 10, e0129768 (2015).
Confavreux, C. et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 13, 247–256 (2014).
Klotz, L. et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl. Med. 11, eaa05563 (2019).
Enouz, S., Carrié, L., Merkler, D., Bevan, M. J. & Zehn, D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J. Exp. Med. 209, 1769–1779 (2012).
Baazim, H. et al. CD8+ T cells induce cachexia during chronic viral infection. Nat. Immunol. 20, 701–710 (2019).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Bannard, O., Kraman, M. & Fearon, D. T. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 323, 505–509 (2009).
Youngblood, B. et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552, 404–409 (2017).
Kim, S. K. et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl Acad. Sci. USA 95, 10814–10819 (1998).
Battegay, M. et al. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33, 191–198 (1991).
Heer, A. K., Harris, N. L., Kopf, M. & Marsland, B. J. CD4+ and CD8+ T cells exhibit differential requirements for CCR7-mediated antigen transport during influenza infection. J. Immunol. 181, 6984–6994 (2008).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Koster, J. & Rahmann, S. Snakemake—a scalable bioinformatics workflow engine. Bioinformatics 34, 3600 (2018).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Ewels, P., Magnusson, M., Lundin, S. & Kaller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Kolde, R. pheatmap: Pretty Heatmaps. r package version 1.0.12 . https://CRAN.R-project.org/package=pheatmap (2019).
Waltman, L & Jan van Eck, Nees. A smart local moving algorithm for large-scale modularity-based community detection. N.J. Eur. Phys. J. B (2013).
Soumillon, M., Cacchiarelli, D., Semrau, S., Oudenaarden, A. & Mikkelsen, T. S. Characterization of directed differentiation by high-throughput single-cell RNA-seq. Preprint at bioRxiv https://doi.org/10.1101/003236 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal https://doi.org/10.14806/ej.17.1.200 (2011).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene-set collection. Cell Syst. 1, 417–425 (2015).
Amezquita, R. A. et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 17, 137–145 (2020).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Yang, X. et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 13, 657–668 (2019).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Angerer, P. et al. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics 32, 1241–1243 (2016).
Ge, H., Xu, K. & Ghahramani, Z. Turing: a language for flexible probabilistic inference. Proc. Mach. Learn. Res. 84, 1682–1690 (2018).
Acknowledgements
We thank B. Youngblood and L. Klotz for input, feedback and suggestions; H. Kohlhof and E. Peelen from Immunic Therapeutics, Gräfelfing, Germany for performing the leflunomide pharmacokinetic; M. J. Bevan, formerly University of Washington, for the permission to use a dataset generated by D.Z. in his laboratory, T. Herbinger, B. Dötterböck, W. Schmid, L. Carrie and C. Amette for technical assistance; and S. Schleicher and C. Lechner for animal husbandry. R. Thimme (University of Freiburg) for the provision of a human tetramer staining protocol. Work in the D.Z. laboratory was supported by a European Research Council starting grant (ProtecTC) and subsequently a European Research Council consolidator grant (ToCCaTa), grants from the Swiss National Science Foundation (CRSII3_160708, 310030E-164187, 51PHP0_157319 and PP00P3_144883), the Swiss Vaccine Research Institute (SVRI), grants from the German Research Foundation (DFG, SFB1054 and SFB1371) and a grant from the German Israeli Foundation (GIF no. 1440). A.M.S. is supported by European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754462, by the DFG (419162346 and SFB1371), the Klaus Tschira Foundation and the German Scholars Organization (KT 34). H.A.M. is a Humboldt Postdoctoral Research Fellow sponsored by the Alexander von Humboldt Foundation. T.K. is supported by the DFG (SFB1054-B06 (ID 210592381), TRR128-A07 (ID 213904703), TRR128-A12 (ID 213904703), TRR128-Z02 (ID 213904703), TRR274-A01 (ID 408885537), TRR355-B07 (ID 490846870) and EXC 2145 (SyNergy, ID 390857198)) and by the Hertie Network of Clinical Neuroscience.
Author information
Authors and Affiliations
Contributions
S.G.O., S.S. and D.Z. initiated the study and made the primary observation. S.S., A.M.S. and D.Z. conceptualized and coordinated the full study, analyzed the data and wrote the paper, and A.M.S. and D.Z. acquired funding. S.S., S.G.O. and K.K. contributed to the design of the study. S.S. and K.K. performed the core experiments and analyzed data, while initial experiments were performed by S.G.O. Additional major experiments and data analysis were performed by A.-K.G., A.M.S., D.Z., H.A.M., J.B., L.A., L.V.D., M.v.H., T.C. and Z.E. S.S., A.M.S., C.W. and A.-K.G. generated transcriptome data. K.K., M.W., P.R., T.N., T.H. and G.P.A. performed computational and statistical analyses of the sequencing data. T.N. and T.H. performed mathematical modeling based on experimental data generated by S.S. I.K., M.J.G.T.V. and C.A.M. selected and recruited participants and L.V.D. processed and immunophenotyped the human blood. D.J.P., F.B., A.G., N.B.B., P.K., M.K., V.F., M.I., M.P., T.K. and E.L.P. provided important scientific input.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Katherine Kedzierska and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
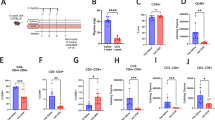
Extended Data Fig. 1 Phenotypes of T cells in DHODH inhibitor treated patients.
A–D, peripheral mononuclear cells were obtained from HLA-A2 positive patients with multiple sclerosis that were long-term treated with teriflunomide (Tfl) or without (Ctrl). Donors were stained with HLA-A2 multimers loaded with the EBV peptide GLCTLVAML (A, C) or CMV NLVPMVATV (B, D) peptide. Shown are the frequencies of multimer positive cells among total CD8+ T cells (A, B) and the percentages of multimer positive CD8+ T cells that express CD127 (C, D). E and F, blood samples from healthy controls (Ctrl) and Teriflunomide (Tfl) treated patients were obtained 14 days after applying the 2nd dose of the Bnt162b2 mRNA vaccine. Isolated PBMC’s were stimulated with overlapping peptide pools of the SARS-CoV-2 spike protein. Shown are the frequencies of IFNγ positive CD4+ T cells that bear a CD45RA- /CCR7−effector memory (TEM) phenotype (E) or CD45RA− /CCR7+ central memory (TCM) phenotype (F). n = 8 for Ctrl or n = 10 for Tfl treated patients. Symbols represent throughout individual patients and the line the mean of the group. Two-tailed, unpaired t-tests were performed to calculate significance with *p < 0.05, **p < 0.01, and ns=not significant (p > 0.05). Supplementary Fig. 7−9 contain gating information.
Extended Data Fig. 2 Suppression of pyrimidine synthesis blocks effector T cells in various infections.
A-E, similarly as indicated in Fig. 1, mice received a low dose of CD45.1 congenic OT-1 or P14, Leflunomide (Lefl) or Teriflunomide (Tfl), and either an infection with Lm-Ova, Ovalbumin expressing Influenza (Flu-OVA), Ovalbumin expressing Vesicular stomatitis virus (VSV-OVA), wildtype Lymphocytic choriomeningitis virus (LCMV), and recombinant Listeria monocytogenes expressing Ovalbumin encoding the high affinity ligand (Lm-N4) or a low affinity altered peptide ligand (Lm-T4). A, frequency of OT-1 T cells among total blood CD8+ T cells 61 days post Lm-Ova infection. B, memory OT-1 T cells were isolated from a different experiment from control and Leflunomide treated mice on day 63 and transferred into naïve hosts. These hosts were then infected with Lm-Ova. Depicted are secondary memory OT-1 at 48 days after the Lm-Ova challenge (note that data are derived from the same experiment shown in Fig. 1f). The further plots show: C, the frequencies of OT-1 among total splenic CD8+ T cells on day 7 after the indicated infections, D, after high or low affinity stimulation, and E, under Teriflunomide instead of Leflunomide treatment in an Lm-Ova infection. F, Teriflunomide plasma levels determined over a 28-day period in mice that were treated with Leflunomide as indicated in Fig. 1a. G, Pharmacokinetic of Teriflunomide in the blood one day after a single dose injection of Leflunomide (Blood Day 1), or on day 7 after applying the treatment regime shown in the scheme (Blood Day 7). H, corresponding to the setup explained in A-E, day 7 OT-1 T cells obtained from different organs from Lm-Ova infected mice were analyzed for KLRG1 and CD127 expression. I, similar analysis for day 7 splenic OT-1 or P14 obtained from the indicated infections. J, spleens and livers from Lm-Ova infected Leflunomide treated and control mice were analyzed for bacteria load on day 7 post infection. Symbols represent individual mice, the line the mean of a group. A linear regression analysis is shown in F. Symbols in F and G show the mean of a group and error bars represent standard deviation (SD). n = 3 (F and G), or 5-10 (A-E and H-J) mice per group. All infection experiments were performed at least two times. Two-tailed, unpaired t-tests were performed to calculate significance with *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns=not significant (p > 0.05). Supplementary Fig. 10 contains gating information.
Extended Data Fig. 3 Pyrimidine starvation confines antigen-specific T cells to the splenic T cell zone.
Mice were engrafted with a low number of CD45.1+ congenic OT-1 T cells, infected with Lm-Ova, and treated with (Lefl) and without Leflunomide (Ctrl). Spleens were harvested 7 days post infection. A, upper panels show splenic sections stained with B220 (blue), CD3 (green), and CD45.1 (red). Lower panels show vectored images, which display the localization of individual OT-1 in red. B, the graphs show the relative distribution of OT-1 in the three anatomical locations and the total OT-1 numbers per mm2 in the indicated anatomical location. Data points represent individual mice, center line shows the mean. Data are representative of n = 3 (Ctrl) and n = 3 (Lefl) mice. Two-tailed, unpaired t-tests were performed to calculate significance with *p < 0.05, ** p < 0.01, and ns=not significant (p > 0.05).
Extended Data Fig. 4 Extended phenotyping of OT-1 T cells activated in the presence of leflunomide.
A, schematic representation of the experimental setup and treatment scheme: Naïve control (Ctrl) or leflunomide (Lefl STOP Day 7 and Lefl continuous) treated host mice received 1 × 104 naïve OT-1 and were infected with Lm-Ova. Data graph shows the frequency of KLRG1+ CD127− OT-1 at 5, 8, 11 and 21 days post infection. B, C, OT-1 T cells isolated at 7 and 35 days post infection from control (Ctrl) and leflunomide (Lefl) treated Lm-Ova infected mice. Cells were briefly ex vivo re-stimulated with Ova-peptide in the presence of brefeldin A and then stained intracellularly for IFNγ and TNF. Representative dot plots are shown. The scatter plots depict all mice per group in the shown representative experiment. D-F, Naïve host mice received a low number of naïve OT-1 and the hosts were infected with Lm-Ova. Cells from the liver were recovered at 29 days post infection post liver perfusion. Data graphs showing the frequencies of OT-1 among total CD8+ T cells and total OT-1 numbers in the liver (D). Representative flow cytometry dot plots and data graphs showing the frequencies of CXCR6+ CD69+ OT-1 on day 29 post infection (E) and the total number of total CXCR6+ CD69+ OT-1 in the liver (F). The scatter plots depict all mice per group in the shown representative experiment. Symbols in A represent the mean of the group, in B-F individual mice and the lines the mean of a group. Error bars in A show the standard deviation (SD). n = 5 mice per group in A-C and n = 4 (Ctrl) and n = 5 (Lefl) mice in D-F. Data are representative of 2 independent experiments. Two-tailed, unpaired t-tests were performed to calculate significance with ***p < 0.001, and ns=not significant (p > 0.05). Supplementary Fig. 11 contains gating information.
Extended Data Fig. 5 Leflunomide reduces endogenous, pathogen-specific effector T cells.
Naïve host mice were infected with LCMV Armstrong and the endogenous T cell response in the spleen was analyzed using Tetramer-gp33 staining on day 8 and day 29 post infection. A, representative flow cytometry dot plots and data graphs showing the frequency, and total numbers, of splenic Tetramer-gp33+ CD8+ T cells on day 8 post infection. B, representative flow cytometry dot plots and data graphs showing the frequencies of KLRG1+ CD127- and CD127+ KLRG1− T cells within the Tetramer+ population on day 8 post infection. C, data graphs showing the total number of KLRG1+ CD127− and CD127+ KLRG1−T cells within the Tetramer+ population on day 8 post infection. The arrows and values indicate the fold reduction of T cell numbers in Leflunomide treated group as compared to the control treated group. D, representative flow cytometry dot plots and data graphs showing the frequency and total numbers of splenic Tetramer+ T cells on day 29 post infection. E, Representative flow cytometry dot plots and data graphs showing the frequencies of KLRG1+ CD127− and CD127+ KLRG1− T cells within the Tetramer+ population on day 29 post infection. F, data graphs show the total number of KLRG1+ CD127− and CD127+ KLRG1−T cells within the Tetramer+ population on day 29 post infection. G, H, representative flow cytometry plots and data graphs showing the frequencies (G) and total numbers (H) of CD44+ CD62L− and CD44+ CD62L+ T cells within the Tetramer+ population on day 29 post infection. The scatter plots depict all mice per group, with n = 5. Symbols represent throughout individual mice and the line the mean of a group. Two-tailed, unpaired t-tests were performed to calculate significance with *p < 0.05, *** p < 0.001, ****p < 0.0001, and ns=not significant (p > 0.05). Supplementary Fig. 12 contains gating information.
Extended Data Fig. 6 T cells from leflunomide treated and untreated mice secrete cytokines similarly in recall responses.
A, schematic representation of the experimental setup of the recall experiment: Primary, naïve control (Ctrl) or leflunomide (Lefl) treated host mice received a low number of naïve OT-1 and were infected with Lm-Ova. After 28 days, memory OT-1 were recovered from the spleen and transferred into untreated, naïve secondary hosts, which were subsequently infected with Lm-Ova. B, data graphs show the frequencies of OT-1 among total CD8+ T cells, and total OT-1 numbers, recovered from the spleen of secondary host mice on day 7 post infection. C, D, representative flow cytometry dot plots and data graphs showing the frequencies (C) and numbers (D) of KLRG1+ CD127− and CD127+ KLRG1− OT-1 from secondary host mice on day 7 post infection. E, representative flow cytometry dot plots and data graphs showing the frequencies of cytokine-producing IFNγ+ and TNF+ OT-1 from secondary host mice on day 7 post Lm-Ova infection, after a brief ex vivo re-stimulation with or without Ova peptide in the presence of brefeldin A followed by intracellular cytokine staining. The scatter plots depict all mice per group. Symbols represent throughout individual mice, lines the mean of a group. n = 5 mice per group. Data are representative of 2 independent experiments. Two-tailed, unpaired t-tests were performed to calculate significance with **p < 0.01 and ****p < 0.0001, and ns=not significant (p > 0.05). Supplementary Fig. 13 contains gating information.
Extended Data Fig. 7 Leflunomide reduces weight in chronic infections and CD4 T cells in acute LCMV infections.
Mice received leflunomide treatments between days -3 and 5 every other day and on day 0 a LCMV docile (A, B), or clone 13 (C, D) infection. A, total numbers of P14 7 days post infection. B, representative flow plots show the frequencies of P14 expressing TIM3 or TCF-1 in spleen and liver. Diagrams show the frequencies of TIM3+ TCF-1- and TCF-1+ TIM3−P14 T cells in spleen (upper panel) and liver (lower panel). C, Diagrams show the frequencies of Tcf1+ P14 (left panel) and TCF-1+CD8+ host cells (left panel) in the blood of mice that have been treated with or without leflunomide every other day. D, body weight curves of up to day 5 treated and untreated LCMV clone 13 infected mice. E, schematic illustration of the experimental setup used in F-I: Naïve control (Ctrl) or leflunomide (Lefl) treated host mice received 3 × 103 SMARTA T cells and were infected with 2 × 105 pfu LCMV Armstrong. Mice were analyzed 8 days post infection. F, data graphs show the frequencies of SMARTA T cells among total CD4+ cells and total SMARTA numbers in the spleen. G, representative flow cytometry plots and data graphs showing the frequencies and H, total numbers of SLAM+CXCR5− Th1 and SLAM−CXCR5+ Tfh cells. I, representative flow cytometry dot plots and data graphs showing the frequencies and numbers of cytokine-producing IFNγ+IL-2+ SMARTA T cells. The scatter plots depict all mice per group. Symbols in A-C and F-I represent individual mice and in D the mean of a group. Error bars in D represent the standard error of the mean of five biological replicates. The line in A-C and F-I represents the mean of the group. n = 5 (A, B and F-I) and n = 6 (C) mice per group. Two-tailed, unpaired t-tests were performed to calculate significance with *p < 0.05, **p < 0.01, ***p = 0.001, ****p = 0.0001, and ns=not significant (p > 0.05). Supplementary Fig. 14 contains gating information.
Extended Data Fig. 8 OT-1 T cells expansion kinetics and DC maturation under leflunomide treatment.
A, schematic representation of the experimental setup: Primary, naïve control (Ctrl) or leflunomide (Lefl) treated host mice received 3 × 105 (Day 1.5), 1 × 105 (Day 3 and Day 4), or 2 × 104 (Day 5) naïve OT-1 and were infected with Lm-Ova. Data graphs show the frequency of KLRG1+CD127− OT-1 on Day 1.5, 3, 4 and 5 post infection. B, in order to assess the time point, when OT-1 start to proliferate in a Listeria infection, naïve host mice received 106 naïve, CFSE labeled OT-1 and the hosts were infected with Lm-Ova. OT-1 were recovered at 36 and 60 h post infection from spleen and analyzed by flow cytometry. Histograms show representative CFSE dilution profiles, where numbers indicate the division. Scatter plots in (A) depict all mice per group in the shown representative experiment. C, relative expression of DHODH mRNA relative to 18 S mRNA in CD8+ T cells sorted for Ametrine expression 48 hours after transduction with either the retroviral construct for Scrambled, DHODH shRNA 1, 2 or 1 + 2 as determined by qPCR. The scatter plot depicts three technical replicates per group in the shown representative experiment. D-F, mice were treated without (Ctrl) or with leflunomide (Lefl), infected with LCMV Armstrong and analyzed for TCR signaling in Nur77 transgenic P14 T cells 1.5 days post infection. Data graphs show the percentages of CD69+ (D), Nur77+ (E) and CD62Lhi (F) P14. G, splenic DCs were analyzed for the activation markers CD80 and MHCII from control (Ctrl) and leflunomide (Lefl) treated mice 2 days post Lm-OVA infection. Data in A and B are representative for at least 2 individual experiments with n = 5 mice (A) and n = 3 mice (B) per group. Symbols in C represent technical replicates or individual mice (D-G), and the line the mean of a group (D-G). n = 15 mice per group for infected Ctrl and Lefl and n = 10 mice per group for Ctrl naïve and Lefl naïve (D-F), and n = 3 mice per group (G). Two-tailed, unpaired t-tests were performed to calculate significance with ***p = 0.001, ****p = 0.0001, and ns=not significant (p > 0.05). Supplementary Fig. 15 contains gating information.
Extended Data Fig. 9 Single-cell transcriptomic data of leflunomide treated and untreated cells.
The analysis relates to Fig. 5. A, heatmap depicting the cluster specific expression of selected genes among the clusters. B, tSNE plots with overlaid expression of predicted upstream regulators in red. Each dot represents a cell. Bar graphs show the percentage of cells expressing the respective upstream regulators in each predicted cluster. C, plots depict an enrichment analysis for gene sets controlled by upstream regulators. p-values were adjusted using Benjamini & Hochberg method. D, heatmap shows the cluster resolved activity of the top 30 upstream regulators. Upstream regulators were in both cases predicted by Ingenuity Pathway Analysis (QIAGEN IPA).
Extended Data Fig. 10 Reproduction of single-cell transcriptomic data after leflunomide treated.
A and B, louvain clusters depicted in the reduced space calculated by UMAP for control (Ctrl) and leflunomide (Lefl) treated mice from 4 biological replicates each. Overlays of all 4 replicates (A) and individual sample (B) data are presented. C, frequencies of the respective clusters of OT-1 T cells defined in A within in the total CD8+ population. D, frequencies of cells expressing the indicated genes (circle size) and their respective scaled expression levels (color intensity) in each cluster. E, Dhodh expression levels depicted over the reduced space calculated by UMAP for control (Ctrl) and leflunomide (Lefl) treated mice. F, frequency histogram representing the distribution of Dhodh expression levels in cells from control (Ctrl) and leflunomide (Lefl) treated mice. G, developmental trajectories for effector and memory branches depicted over the diffusion map for each condition. Cells are colored according to the clustering. Each dot in A, B, E and G represents a cell. Symbols in C represent individual mice, n = 4 mice per group, and the conditions in C were compared for each cluster using two-tailed, unpaired t-tests with *p < 0.05, **p < 0.01, and ns=not significant (p > 0.05).
Supplementary information
Supplementary Information
Supplementary Figs. 1–15
Source data
Source Data Fig. 1
Excel file with raw data for graphical items.
Source Data Fig. 2
Excel file with raw data for graphical items.
Source Data Fig. 3
Excel file with raw data for graphical items.
Source Data Fig. 4
Excel file with raw data for graphical items.
Source Data Fig. 5
Excel file with raw data for graphical items.
Source Data Fig. 6
Excel file with raw data for graphical items.
Source Data Fig. 7
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 1
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 2
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 3
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 4
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 5
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 6
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 7
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 8
Excel file with raw data for graphical items.
Source Data Extended Data Fig./Table 10
Excel file with raw data for graphical items.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scherer, S., Oberle, S.G., Kanev, K. et al. Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development. Nat Immunol 24, 501–515 (2023). https://doi.org/10.1038/s41590-023-01436-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-023-01436-x
This article is cited by
-
DHODH modulates immune evasion of cancer cells via CDP-Choline dependent regulation of phospholipid metabolism and ferroptosis
Nature Communications (2025)
-
A Comprehensive Stability-indicating Method for Teriflunomide: Impurity Profiling and Degradation Analysis in Tablet Formulations
Chromatographia (2025)
-
Inhibition of ENT1 relieves intracellular adenosine-mediated T cell suppression in cancer
Nature Immunology (2025)
-
Inactivation of cytidine triphosphate synthase 1 prevents fatal auto-immunity in mice
Nature Communications (2024)
-
Extracellular domains of CARs reprogramme T cell metabolism without antigen stimulation
Nature Metabolism (2024)