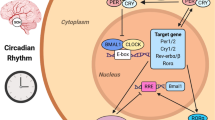
Abstract
The circadian clock is a critical regulator of immunity, and this circadian control of immune modulation has an essential function in host defense and tumor immunosurveillance. Here we use a single-cell RNA sequencing approach and a genetic model of colorectal cancer to identify clock-dependent changes to the immune landscape that control the abundance of immunosuppressive cells and consequent suppression of cytotoxic CD8+ T cells. Of these immunosuppressive cell types, PD-L1-expressing myeloid-derived suppressor cells (MDSCs) peak in abundance in a rhythmic manner. Disruption of the epithelial cell clock regulates the secretion of cytokines that promote heightened inflammation, recruitment of neutrophils and the subsequent development of MDSCs. We also show that time-of-day anti-PD-L1 delivery is most effective when synchronized with the abundance of immunosuppressive MDSCs. Collectively, these data indicate that circadian gating of tumor immunosuppression informs the timing and efficacy of immune checkpoint inhibitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
scRNA-seq data have been deposited at the NCBI Gene Expression Omnibus under accession code GSE262267 and in the Sequence Read Archive under accession code PRJNA1003452. Source data are provided with this paper.
References
Sinicrope, F. A. Increasing incidence of early-onset colorectal cancer. N. Engl. J. Med. 386, 1547–1558 (2022).
Ben-Aharon, I. et al. Early-onset cancer in the gastrointestinal tract is on the rise—evidence and implications. Cancer Discov. 13, 538–551 (2023).
Meulendijks, D. et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 16, 1639–1650 (2015).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Diaz, L. A. et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 23, 659–670 (2022).
André, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Antonia, S. J. et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 20, 1395–1408 (2019).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Bass, J. & Lazar, M. A. Circadian time signatures of fitness and disease. Science 354, 994–999 (2016).
Scheiermann, C., Gibbs, J., Ince, L. & Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 18, 423–437 (2018).
Benitah, S. A. & Welz, P.-S. Circadian regulation of adult stem cell homeostasis and aging. Cell Stem Cell 26, 817–831 (2020).
Gibbs, J. et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 20, 919–926 (2014).
Méndez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P. S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008).
Curtis, A. M., Bellet, M. M., Sassone-Corsi, P. & O’Neill, L. A. J. Circadian clock proteins and immunity. Immunity 40, 178–186 (2014).
Gibbs, J. et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 582–587 (2012).
Sutton, C. E. et al. Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat. Commun. 8, 1923 (2017).
Nguyen, K. D. et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341, 1483–1488 (2013).
Hadadi, E. et al. Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat. Commun. 11, 3193 (2020).
Wang, C. et al. Dendritic cells direct circadian anti-tumor immune responses. Nature 614, 136–143 (2022).
Lévi, F. A. et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J. Natl Cancer Inst. 86, 1608–1617 (1994).
Lévi, F., Zidani, R. & Misset, J. L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350, 681–686 (1997).
Gou, X. X. et al. Induction chronomodulated chemotherapy plus radiotherapy for nasopharyngeal carcinoma: a phase II prospective randomized study. J. Cancer Res. Ther. 14, 1613–1619 (2018).
Iacobelli, S. et al. A phase I study of recombinant interferon-α administered as a seven-day continuous venous infusion at circadian-rhythm modulated rate in patients with cancer. Am. J. Clin. Oncol. 18, 27–31 (1995).
Deprés-Brummer, P. et al. A phase I trial of 21-day continuous venous infusion of α-interferon at circadian rhythm modulated rate in cancer patients. J. Immunother. 10, 440–447 (1991).
Re, G. L. et al. Interleukin-2 chronotherapy for metastatic renal cell carcinoma: results of a phase I-II study. Cytokine 128, 154984 (2020).
Qian, D. C. et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 22, 1777–1786 (2021).
Landre, T. et al. Time-dependent efficacy of immune checkpoint inhibitors in the treatment of metastatic cancers: a meta-analysis. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.2562 (2023).
Karaboué, A. et al. Time-dependent efficacy of checkpoint inhibitor nivolumab: results from a pilot study in patients with metastatic non-small-cell lung cancer. Cancers 14, 896 (2022).
Yeung, C., Kartolo, A., Tong, J., Hopman, W. & Baetz, T. Association of circadian timing of initial infusions of immune checkpoint inhibitors with survival in advanced melanoma. Immunotherapy 15, 819–826 (2023).
England, C. G. et al. Preclinical pharmacokinetics and biodistribution studies of 89Zr-labeled pembrolizumab. J. Nucl. Med. 58, 162–168 (2017).
Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016).
Kettner, N. M. et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924 (2016).
Lee, Donehower, L. A., Herron, A. J., Moore, D. D. & Fu, L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE 5, e10995 (2010).
Stokes, K. et al. The circadian clock gene, Bmal1, regulates intestinal stem cell signaling and represses tumor initiation. Cell. Mol. Gastroenterol. Hepatol. 12, 1847–1872 (2021).
Pariollaud, M. et al. Circadian disruption enhances HSF1 signaling and tumorigenesis in Kras-driven lung cancer. Sci. Adv. https://doi.org/10.1126/sciadv.abo1123 (2022).
Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002).
Wood, P. A. et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol. Cancer Res. 6, 1786–1793 (2008).
Chun, S. K. et al. Disruption of the circadian clock drives Apc loss of heterozygosity to accelerate colorectal cancer. Sci. Adv. 8, 2389 (2022).
Fekry, B. et al. Incompatibility of the circadian protein BMAL1 and HNF4α in hepatocellular carcinoma. Nat. Commun. 9, 4349 (2018).
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018b).
Dong, Z. et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 9, 1556–1573 (2019).
Chen, P. et al. Circadian regulator CLOCK recruits immune-suppressive microglia into the GBM tumor microenvironment. Cancer Discov. 10, 371–381 (2020).
Cheung, A. F. et al. Complete deletion of Apc results in severe polyposis in mice. Oncogene 29, 1857–1864 (2010).
Wei, Y. et al. B cell heterogeneity, plasticity, and functional diversity in cancer microenvironments. Oncogene 40, 4737–4745 (2021).
Liu, R.-X. et al. Altered B cell immunoglobulin signature exhibits potential diagnostic values in human colorectal cancer. iScience 26, 106140 (2023).
Shalapour, S. & Karin, M. The neglected brothers come of age: B cells and cancer. Semin. Immunol. 52, 101479 (2021).
Oosterman, J. E., Wopereis, S. & Kalsbeek, A. The circadian clock, shift work, and tissue-specific insulin resistance. Endocrinology 161, bqaa180 (2020).
Aiello, I. et al. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 6, eaaz4530 (2020).
Alshetaiwi, H. et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 5, eaay6017 (2020).
Huang, B. et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66, 1123–1131 (2006).
Zhao, H. et al. Myeloid-derived itaconate suppresses cytotoxic CD8+ T cells and promotes tumour growth. Nat. Metab. 4, 1660–1673 (2022).
Jou, E. et al. An innate IL-25–ILC2–MDSC axis creates a cancer-permissive microenvironment for Apc mutation–driven intestinal tumorigenesis. Sci. Immunol. 7, eabn0175 (2022).
Youn, J.-I., Nagaraj, S., Collazo, M. & Gabrilovich, D. I. Subsets of myeloid-derived suppressor cells in tumor bearing mice. J. Immunol. 181, 5791–5802 (2008).
Kusmartsev, S. A., Li, Y. & Chen, S.-H. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation1. J. Immunol. 165, 779–785 (2000).
Klement, J. D. et al. Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment. Cancer Cell 41, 620–636.e9 (2023).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Nusse, R. & Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Casey, S. C. et al. MYC regulates the anti-tumor immune response through CD47 and PD-L1. Science 352, 227–231 (2016).
Kortlever, R. M. et al. Myc cooperates with Ras by programming inflammation and immune suppression. Cell 171, 1301–1315 (2017).
Kozuka, K. et al. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep. 9, 1976–1990 (2017).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Zhao, H. et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 6, 1–46 (2021).
Ban, Y. et al. Targeting autocrine CCL5–CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 77, 2857–2868 (2017).
Ernst, M. & Putoczki, T. IL-17 cuts to the chase in colon cancer. Immunity 41, 880–882 (2014).
Chung, A. S. et al. An interleukin-17–mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 19, 1114–1123 (2013).
Borish, L. C. & Steinke, J. W. Cytokines and chemokines. J. Allergy Clin. Immunol. 111, S460–S475 (2003). 2.
Kohli, K., Pillarisetty, V. G. & Kim, T. S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 29, 10–21 (2022).
Santos, I. et al. CXCL5-mediated recruitment of neutrophils into the peritoneal cavity of Gdf15-deficient mice protects against abdominal sepsis. Proc. Natl Acad. Sci. USA 117, 12281–12287 (2020).
Besnard, A.-G. et al. CXCL6 antibody neutralization prevents lung inflammation and fibrosis in mice in the bleomycin model. J. Leukoc. Biol. 94, 1317–1323 (2013).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752.e20 (2021).
Zhang, B. et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE 8, e57114 (2013).
Karakasheva, T. A. et al. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight 3, e97022 (2018).
Huang, T. et al. Wnt inhibition sensitizes PD-L1 blockade therapy by overcoming bone marrow-derived myofibroblasts-mediated immune resistance in tumors. Front. Immunol. 12, 619209 (2021).
Pilato, M. D. et al. Targeting the CBM complex causes Treg cells to prime tumors for immune checkpoint therapy. Nature 570, 112–116 (2019).
Roberts, N. T., MacDonald, C. R., Mohammadpour, H., Antoch, M. P. & Repasky, E. A. Circadian rhythm disruption increases tumor growth rate and accumulation of myeloid‐derived suppressor cells. Adv. Biol. 6, 2200031 (2022).
Cervantes-Silva, M. P. et al. The circadian clock influences T cell responses to vaccination by regulating dendritic cell antigen processing. Nat. Commun. 13, 7217 (2022).
Diamantopoulou, Z. et al. The metastatic spread of breast cancer accelerates during sleep. Nature 607, 156–162 (2022).
Ferris, R. L. et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 31, 942–950 (2020).
Garg, A. D. et al. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. Oncoimmunology 6, e1295903 (2017).
Storch, K.-F. et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell 130, 730–741 (2007).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Acknowledgements
B.M.F. and S.M.P. were supported by the National Cancer Institute (NCI) T32 Interdisciplinary Cancer Research Training Program (grant no. T32CA009054) and B.M.F. was also supported by the NCI grant F31CA287992. A.L.M. was supported by the National Science Foundation Graduate Research Fellowship Program (grant no. DGE1839285). A.M. was supported by the NCI grant F31AR083279. We acknowledge the support of the Chao Family Comprehensive Cancer Center at the University of California, Irvine, which is supported by the NCI (P30 CA062203). Shared resources included use of the Genomics Research and Technology Hub. We thank the Institute for Immunology Flow Cytometry Facility and the Stem Cell Flow Core at the University of California, Irvine for technical assistance. The Pannunzio laboratory is supported by National Institutes of Health (NIH)/NCI grants R37CA266042 and R01CA276470. The Seldin laboratory is supported by the NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant DP1DK130640. The Marangoni laboratory is supported by the Melanoma Research Alliance Bristol Meyers Squibb Young Investigator Award no. 929155 and the DoD Team grant ME220176P1. Financial support for the Masri laboratory is provided through the NIH/NCI (R01CA244519 and R01CA259370), the V Foundation for Cancer Research and Johnson & Johnson.
Author information
Authors and Affiliations
Contributions
Conceptualization was the responsibility of B.M.F. and S.M. Methodology was the responsibility of B.M.F., J.I.R., H.A., O.S.E., N.R.P., M.M.S., I.M., F.M., D.A.L., and K.K. Investigation was carried out by B.M.F., S.M.P., J.I.R., H.A., A.N.L., A.M., W.A.S., A.L.M., S.K.C., A.H., I.A. and M.M.S. Visualization was carried out by B.M.F. and S.M.P. Supervision was carried out by S.M. B.M.F., S.M.P. and S.M. were responsible for writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Dmitry Gabrilovich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Circadian clock disruption promotes intestinal tumorigenesis.
(a) Graphic of mouse genotypes. (b) Gene targeting strategy for generation of intestine-specific knockout of Bmal1 and Apc. (c) Small intestinal polyp count from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice (n = 15 mice/genotype). (d) Spleen weight from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice (n = 15 mice/genotype). (e) Violin plots of RNA features, count, and percent of mitochondrial RNA for each immune cell cluster prior to filtering mitochondrial RNA < 10% and nFeature < 3000. (f) Heatmap of top three genes expressed by each immune cell cluster. Data represent the mean ± SEM and statistical significance was determined by one-way ANOVA with Tukey’s multiple comparison test for C and D. Asterisks represent p-values from multiple comparisons, with **** indicating a p-value of < 0.0001.
Extended Data Fig. 2 scRNA-seq demonstrates that clock disruption alters the immune landscape.
a) Dot-plot of gene expression by each immune cell cluster. (b) Pie chart of CD25+ T cells and CD3+ T cells from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice as determined by scRNA-seq. (c) Bar graph of immune cell count for neutrophils, monocytes/macrophages, and dendritic cells (DCs) from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice as determined by scRNA-seq. (d) Bar graph of immune cell count for naïve B cells, proliferating B cells, mature B cells, CD8+ T cells, CD4+ T cells, and CD25+ T cells from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice as determined by scRNA-seq. (e) Dot-plot of gene expression for B cell clusters.
Extended Data Fig. 3 Gating strategies for flow cytometric analysis of the immune landscape.
(a) Gating strategy for flow cytometric analysis of immune cells. Markers include Sytox Blue, APC CD45, PerCP-Cy7 CD11b, PE F4/80, FITC Ly6G, BV605 Ly6C, PE CD3, FITC CD8, APC-Cy7 CD4, and BV605 CD25. (b) Gating strategy for sorting live, CD45+CD11b+Gr1+ cells. Markers include Sytox Blue, APC CD45, PE Gr1, and FITC CD11b. (c) Gating strategy for T cells post co-culture with CD11b+Gr1+ cells. Markers include FITC Zombie, PE-Cy7 CD8, and DAPI CD4.
Extended Data Fig. 4 Flow cytometry demonstrates that clock disruption alters immune proportions.
(a) Bar graph of immune cells as percent of live cells from the intestine of WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype). (b) Bar graph of immune cell counts for neutrophils, monocytes, and macrophages from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype). (c) Bar graph of immune cell counts for CD8+ T cells and CD4+ T cells from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype). (d) Bar graph of immune cells as percent of live for neutrophils, monocytes, and macrophages from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype). (e) Bar graph of immune cells as percent of live for CD4+ T cells and CD8+ T cells from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype).
Extended Data Fig. 5 Genetic and environmental clock disruption alter the immune landscape.
(a) CD3+ T cells, CD4+ T cells, and CD25+ T cells shown as percent of CD45+ cells from the small intestine of WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype). (b) Neutrophils, monocytes, macrophages, CD8+ T cells, and CD25+ T cells shown as percent of CD45+ cells from the spleen of WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and analyzed by flow cytometry (n = 7 mice/genotype). (c) Spleen weight of WT mice subjected to 12:12 LD paradigm versus SD (n = 10 mice/genotype). (d) CD4+ T cells shown as percent of CD45+ cells from the small intestine of WT mice subjected to 12:12 LD versus SD. Mice were sacrificed at ZT 4 and analyzed by flow cytometry (n = 8 mice/genotype). (e) Spleen weight of WT mice subjected to 12:12 LD paradigm, 1 week SD, 3 weeks SD, and 5 weeks SD (n = 6 mice/genotype). (f) CD4+ T cells shown as percent of CD45+ cells from the small intestine of WT mice subjected to 12:12 LD paradigm, 1 week SD, 3 weeks SD, and 5 weeks SD. Mice were sacrificed at ZT 4 and analyzed by flow cytometry (n = 6 mice/genotype). Data represent the mean ± SEM and statistical significance was determined by two-tailed Mann-Whitney T-test for A–D, and one-way ANOVA with Tukey’s multiple comparison test for E and F. Asterisks represent p-values from multiple comparisons, with * indicating a p-value of < 0.05, ** indicating a p-value of < 0.01, **** indicating a p-value of < 0.0001, and ns = not significant.
Extended Data Fig. 6 scRNA-seq demonstrates that clock disruption promotes MDSC accumulation.
(a) UMAP of the expression of Ifitm1, Wfdc17, s100a8, s100a9, Irg1, and Arg2 by monocytes/macrophages, neutrophils, and DCs. (b) UMAP of monocytes/macrophages, neutrophils, and DCs in WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice as determined by scRNA-seq. c) Histogram of CD4+ T cell and CD8+ T cell counts after co-culture with Gr1+ cells sorted from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice spleen. (d) Counts of generation 1, 2, and 3 CD4+ T cells and CD8+ T cells after co-culture with Gr1+ cells sorted from WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice spleen (n = 3 mice/genotype). Data represent the mean ± SEM and statistical significance was determined by one-way ANOVA with Tukey’s multiple comparison test for D. Asterisks represent p-values from multiple comparisons, with ** indicating a p-value of < 0.01, *** indicating a p-value of < 0.001, **** indicating a p-value of < 0.0001, and ns = not significant.
Extended Data Fig. 7 Circadian clock disruption promotes an inflammatory response.
(a) Western blot of MYC and p84 in WT and Bmal1−/− intestinal organoids untreated or treated with 100 ng/mL recombinant Wnt3a for 72 hours. (b) WT and Bmal1−/− intestinal organoids untreated or treated with 25, 50, or 100 ng/mL recombinant Wnt3a for 72 hours. Gene expression of c-Myc, Survivin, and Cxcl5 was determined by qPCR (n = 3 independent organoid lines/genotype). (c) WT and Bmal1−/− intestinal organoids untreated or treated with 100 ng/mL recombinant Wnt3a for 24, 48, or 72 hours. Gene expression of c-Myc, Survivin and Cxcl5 was determined by qPCR (n = 3 independent organoid lines/genotype). (d) Concentration of CCL5, IL-17, and CXCL9 in WT and Bmal1−/− intestinal monolayer lysate as determined by ELISA (n = 3 independent monolayer lines/genotype). (e) WT intestinal monolayers were left untreated or treated with 100 ng/mL recombinant Wnt3a for 96 hours (n = 3 untreated and 5 Wnt3a-treated independent monolayer lines/genotype). Concentration of CXCL1, G-CSF, M-CSF, GM-CSF, IL-2, IFNγ, CXCL2, and TNF in WT monolayer cell lysate was determined by ELISA. Data represent the mean ± SEM and statistical significance was determined by two-tailed Mann-Whitney T-test for D, and one-way ANOVA with Tukey’s multiple comparison test for B-C, and E. Asterisks represent p-values from multiple comparisons, with * indicating a p-value of < 0.05, ** indicating a p-value of < 0.01, *** indicating a p-value of < 0.001, **** indicating a p-value of < 0.0001, and ns = not significant.
Extended Data Fig. 8 Wnt signaling mediates the inflammatory response in the intestine.
(a) WT and Bmal1−/− intestinal monolayers were left untreated or treated with 100 ng/mL recombinant Wnt3a for 96 hours (n = 3 untreated and 4 Wnt3a-treated independent monolayer lines/genotype). Concentration of CXCL5, CXCL6, and CXCL2 in intestinal monolayer lysate as determined by ELISA. (b) Expression of c-Myc, Survivin, Axin2, Cxcl5, Cxcl1, M-csf, and Gm-csf as determined by qPCR in mouse embryonic fibroblasts untreated or treated with 50, 100, or 200 ng/mL recombinant Wnt3a for 4 hours (n = 3 independent biological replicates/condition). (c) Expression of c-Myc, Survivin, Cxcl5, and Cxcl1 as determined by qPCR using WT intestinal monolayers infected with shEV or shMyc (n = 3 independent monolayer lines). Data represent the mean ± SEM and statistical significance was determined by two-tailed Mann-Whitney T-test for C, and one-way ANOVA with Tukey’s multiple comparison test for A-B. Asterisks represent p-values from multiple comparisons, with * indicating a p-value of < 0.05, ** indicating a p-value of < 0.01, *** indicating a p-value of < 0.001, **** indicating a p-value of < 0.0001, and ns = not significant.
Extended Data Fig. 9 scRNA-seq analysis of Gr1 and PD-L1 abundance in human CRC.
Dataset from Pelka et al. (a) Dot-plot of gene expression by each immune cell cluster in matched normal colon and tumor samples. (b) Heatmap of cell types clustered by single-cell transcriptional analysis (n = 55,535 cells, n = 36 patients). (c) Stacked bar chart of human immune cell cluster composition determined using scRNA-seq dataset from Pelka et al. Data was based on 36 patients with matched normal colon and CRC tumor samples. (d) UMAP of human immune cell types clustered by single-cell transcriptional analysis broken down by normal and tumor (n = 36 patient samples/group). (e) Bar graph of cell counts expressing PD-L1 by human immune cell clusters in matched normal colon and CRC samples. (f) Dot-plot of PD-L1 expression by each human immune cell cluster in matched normal colon and CRC samples. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparison test for E. Asterisks represent p-values from multiple comparisons, with *** indicating a p-value of < 0.001, **** indicating a p-value of < 0.0001, and ns = not significant.
Extended Data Fig. 10 The circadian clock regulates immunosuppression and anti-PD-L1 efficacy.
(a, b) Box plot or dot-plot of PD-L1 expression by each immune cell cluster in mouse scRNA-seq data (n = 3 mice/genotype). Box plot center line represents the median, the boundaries represent IQR, and the whisker length represents 1.5 x IQR. (c) Bar graph of PD-L1 expression by monocyte/macrophage and neutrophil clusters in the intestine of WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice determined by scRNA-seq (n = 3 mice/genotype). (d-e) UMAP of myeloid cell clusters and PD-L1 expression in the intestine of WT, Bmal1−/−, Apc+/−, and Apc+/−;Bmal1−/− mice (n = 3 mice/genotype). (f-g) Small intestinal polyps and spleen weight in Apc+/−;Bmal1−/− mice (n = 7 mice/group). (h-i) Mesenteric lymph node or blood Gr1+ cells as percent of CD45+ cells from WT and Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and ZT 16 and analyzed by flow cytometry (n = 3 mice/genotype for H, n = 4 mice/genotype for I). (j) Combined total Gr1+ cells from the small intestine and spleen as percent of CD45+ cells from Apc+/−;Bmal1−/− mice sacrificed at ZT 4 and ZT 16 and analyzed by flow cytometry (n = 7 mice/group). (k) Combined total Gr1+PD-L1+ cells from the small intestine and spleen as percent of CD45+ cells from Apc+/−;Bmal1−/− mice. Animals were sacrificed at ZT 4 and ZT 16 and analyzed by flow cytometry (n = 5 mice/group). (l) Small intestinal polyp count from Apc+/−;Bmal1−/− mice untreated or treated with anti-PD-L1 (n = 6 mice/group). (m) Tumor volume over time for WT mice after subcutaneous injection of CMT167 cells and treatment with IgG or anti-PD-L1 at ZT 4 or ZT 16 (n = 5 mice/group, 2 tumors/mouse). (n) Tumor volume over time for WT mice after subcutaneous injection of D4M-S cells and treatment with IgG or anti-PD-L1 at ZT 4 or ZT 16 (n = 5 mice/group, 2 tumors/mouse). Data represent the mean ± SEM and statistical significance was determined by two-tailed Mann-Whitney T-test for F-K, and by one-way ANOVA with Tukey’s multiple comparison test for A, C, and L. Asterisks represent p-values from multiple comparisons, with * indicating a p-value of < 0.05, ** indicating a p-value of < 0.01, **** indicating a p-value of < 0.0001, and ns = not significant.
Supplementary information
Supplementary Information
Supplementary Table 1
Source data
Source Data Fig. 4
Unprocessed immunoblots.
Source Data Extended Data Fig. 7
Unprocessed immunoblots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fortin, B.M., Pfeiffer, S.M., Insua-Rodríguez, J. et al. Circadian control of tumor immunosuppression affects efficacy of immune checkpoint blockade. Nat Immunol 25, 1257–1269 (2024). https://doi.org/10.1038/s41590-024-01859-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-024-01859-0
This article is cited by
-
Remodeling the tumor dormancy ecosystem to prevent recurrence and metastasis
Signal Transduction and Targeted Therapy (2026)
-
Circadian fluctuation of soluble CD26 dictates the impact of the timing of cord blood transplantation on acute graft-versus-host disease
Nature Communications (2026)
-
Time-of-day immunochemotherapy in non-small cell lung cancer: a randomized phase 3 trial
Nature Medicine (2026)
-
Turning cold tumors into hot tumors to ignite immunotherapy
Molecular Cancer (2025)
-
Neuroscience of cancer: unraveling the complex interplay between the nervous system, the tumor and the tumor immune microenvironment
Molecular Cancer (2025)