Abstract
To understand the role of T cells in the pathogenesis of ulcerative colitis (UC), we analyzed colonic T cells isolated from patients with UC and controls. Here we identified colonic CD4+ and CD8+ T lymphocyte subsets with gene expression profiles resembling stem-like progenitors, previously reported in several mouse models of autoimmune disease. Stem-like T cells were increased in inflamed areas compared to non-inflamed regions from the same patients. Furthermore, TCR sequence analysis indicated stem-like T cells were clonally related to proinflammatory T cells, suggesting their involvement in sustaining effectors that drive inflammation. Using an adoptive transfer colitis model in mice, we demonstrated that CD4+ T cells deficient in either BCL-6 or TCF1, transcription factors that promote T cell stemness, had decreased colon T cells and diminished pathogenicity. Our results establish a strong association between stem-like T cell populations and UC pathogenesis, highlighting the potential of targeting this population to improve clinical outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw data are publicly available, and GEO links are provided. The RNA-seq datasets have been deposited in the NCBI GEO database under primary accession number GSE235665. For the transcriptomic analysis, reads were mapped to mm10 (v3.0.0) for mouse data and to hg19 (v3.0.0) for human data. Source data are provided with this paper.
Code availability
All codes for bioinformatic analysis were deposited in our GitHub repository (https://github.com/vijaybioinfo/UC_2024).
References
Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Prim. 6, 74 (2020).
de Souza, H. S. P. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27 (2016).
Hirten, R. P. & Sands, B. E. New therapeutics for ulcerative colitis. Annu. Rev. Med. 72, 199–213 (2021).
Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 178, 714–730 (2019).
Corridoni, D. et al. Single-cell atlas of colonic CD8+ T cells in ulcerative colitis. Nat. Med. 26, 1480–1490 (2020).
Boland, B. S. et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci. Immunol. 5, eabb4432 (2020).
Tajima, M. et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J. Exp. Med. 205, 1019–1027 (2008).
Sun, L., Kong, R., Li, H. & Wang, D. The role of T follicular helper cells and interleukin-21 in the pathogenesis of inflammatory bowel disease. Gastroenterol. Res. Pract. 2021, 9621738 (2021).
Schnell, A. et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 184, 6281–6298 (2021).
Grebinoski, S. et al. Autoreactive CD8+ T cells are restrained by an exhaustion-like program that is maintained by LAG3. Nat. Immunol. 23, 868–877 (2022).
Yi, L. & Yang, L. Stem-like T cells and niches: implications in human health and disease. Front. Immunol. 13, 907172 (2022).
Gearty, S. V. et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602, 156–161 (2022).
Karmaus, P. W. et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature 565, 101–105 (2019).
Shin, B. et al. Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation. J. Exp. Med. 215, 1803–1812 (2018).
Zou, D. et al. CD4+ T cell immunity is dependent on an intrinsic stem-like program. Nat. Immunol. 25, 66–76 (2024).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Senju, M., Wu, K. C., Mahida, Y. R. & Jewell, D. P. Coexpression of CD4 and CD8 on peripheral blood T cells and lamina propria T cells in inflammatory bowel disease by two colour immunofluorescence and flow cytometric analysis. Gut 32, 918–922 (1991).
Romero, P. et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 178, 4112–4119 (2007).
Bacher, P. et al. Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cell 167, 1067–1078 (2016).
Rocha-Perugini, V. et al. CD81 controls sustained T cell activation signaling and defines the maturation stages of cognate immunological synapses. Mol. Cell. Biol. 33, 3644–3658 (2013).
Schönle, A. et al. Caveolin-1 regulates TCR signal strength and regulatory T-cell differentiation into alloreactive T cells. Blood 127, 1930–1939 (2016).
Denning, T. L. et al. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J. Immunol. 178, 4230–4239 (2007).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Jonsson, A. H. et al. Granzyme K(+) CD8 T cells form a core population in inflamed human tissue. Sci. Transl. Med. 14, eabo0686 (2022).
Xu, L. et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat. Immunol. 16, 991–999 (2015).
Zhao, X., Shan, Q. & Xue, H. H. TCF1 in T cell immunity: a broadened frontier. Nat. Rev. Immunol. 22, 147–157 (2022).
Wu, T. et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1, eaai8593 (2016).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Yu, J. et al. Interleukin‑21 promotes the development of ulcerative colitis and regulates the proliferation and secretion of follicular T helper cells in the colitides microenvironment. Mol. Med. Rep. 11, 1049–1056 (2015).
Long, Y., Zhao, X., Liu, C., Xia, C. & Liu, C. Activated inducible co-stimulator-positive programmed cell death 1-positive follicular helper T cells indicate disease activity and severity in ulcerative colitis patients. Clin. Exp. Immunol. 202, 106–118 (2020).
Long, Y. et al. The imbalance of circulating follicular helper T cells and follicular regulatory T cells is associated with disease activity in patients with ulcerative colitis. Front. Immunol. 11, 104 (2020).
Xue, G. et al. Aberrant alteration of follicular T helper cells in ulcerative colitis patients and its correlations with interleukin-21 and B cell subsets. Medicine 98, e14757 (2019).
Tian, Y. & Zajac, A. J. IL-21 and T cell differentiation: consider the context. Trends Immunol. 37, 557–568 (2016).
Elsaesser, H., Sauer, K. & Brooks, D. G. IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009).
Yi, J. S., Du, M. & Zajac, A. J. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009).
Fröhlich, A. et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009).
Powrie, F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity 3, 171–174 (1995).
Zhang, R. et al. T follicular helper cells restricted by IRF8 contribute to T cell-mediated inflammation. J. Autoimmun. 96, 113–122 (2019).
Mucida, D. et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 14, 281–289 (2013).
Yang, Q. et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat. Immunol. 16, 1044–1050 (2015).
Yang, L. et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454, 350–352 (2008).
Fina, D. et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 134, 1038–1048 (2008).
Holm, T. L. et al. Evaluating IL-21 as a potential therapeutic target in Crohn’s disease. Gastroenterol. Res. Pract. 2018, 5962624 (2018).
Takeuchi, A. et al. CRTAM determines the CD4+ cytotoxic T lymphocyte lineage. J. Exp. Med. 213, 123–138 (2016).
Chen, L. et al. IFN-gamma+ cytotoxic CD4+ T lymphocytes are involved in the pathogenesis of colitis induced by IL-23 and the food colorant red 40. Cell Mol. Immunol. 19, 777–790 (2022).
Yokoi, T. et al. Identification of a unique subset of tissue-resident memory CD4+ T cells in Crohn’s disease. Proc. Natl Acad. Sci. USA 120, e2204269120 (2023).
Maaser, C. et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 13, 144–164 (2019).
Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G. & Long, M. D. ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413 (2019).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Acknowledgements
We recognize La Jolla Institute Flow Core Facility for their assistance with cell sorting on a FACSAria Fusion Cell Sorter (supported by NIH grant S10 RR027366); K. Dobaczewska, S. McArdle (supported by Chan Zuckerberg Initiative and Silicon Valley Community Foundation) and Z. Mikulski for assistance with microscopy on a Zeiss LSM 880 confocal laser scanning microscope (supported by NIH grant S10OD021831); K. Kim for assistance with colon histology assessment; and H. Simon for assistance with library preparation and next-generation sequencing using Illumina NovaSeq 6000 (supported by NIH grant S10 OD025052). Supported by the De Laszlo Foundation (R.H.) and the William K. Bowes Jr Foundation, Kyowa Kirin, Inc. (KKNA). (P.V.), NIH grant DK46763 (M.K.) and NIH grant F32 AI 140581 (D.G.). The funders have no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.K., P.V., F.C. and T.S.-E. conceived of the work; Y.L. performed and analyzed experiments in mouse model of colitis under the supervision of M.K.; R.H. coordinated clinical assessments, patient recruitment, and collection and processing of colonic samples under the supervision of F.C. and T.S.-E.; A.D. and C.M. coordinated clinical assessments, patient recruitment, and collection and processing of colonic tissue samples for patients with UC and controls under the supervision of T.P.-J.; C.R.-S. and F.E.C.-C. performed bioinformatic analysis under the supervision of G.S. and P.V.; D.A.G., J.C. and R.H. performed sample processing and sorting of T cells from human samples for single-cell transcriptomic analysis; G.A. analyzed immunofluorescence staining under the supervision of M.K.; Y.L. analyzed data under the supervision of M.K. and P.V.; Y.L. wrote the first draft of the paper that was revised by M.K. and P.V.
Corresponding authors
Ethics declarations
Competing interests
P.V. has consulted for Merck and Co., and M.K. has served on the scientific advisory board of Prometheus Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Vassiliki Boussiotis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
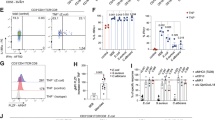
Extended Data Fig. 1 Stem-like T cells are present in colonic tissue.
a, Flow-cytometric plots show sequential gating strategy used for isolating T cells from colonic tissue samples for single-cell RNA-seq, CITE-seq and TCR-seq analyses. b, Violin plots show the per-cell distribution of unique genes, unique molecular identifiers (UMI), and percentage of UMI mapped to mitochondrial genome in T cell clusters in colonic tissue samples (n = 36). Box plots extend from the 25th to 75th percentile and the center line represents the median. Whiskers are bounded by 25th percentile - 1.5*interquartile range or 75th percentile + 1.5*interquartile range. c, UMAP showing Seurat-based clustering of 13,887 T cells (7,047 CD8+, 6,743 CD4+ and 97 cycling T cells), colored based on cluster type; labels on clusters indicate T cell subsets and their assigned cluster identity. d, UMAP illustrating log normalized UMI counts of CD4 and CD8α surface antibody in single cells, measured by CITE-seq (top), and Seurat-normalized expression levels of CD4 and CD8A transcripts in single cells (bottom). e, GSEA plot shows enrichment of indicated gene signatures in the indicated cell clusters compared to the rest of the CD4+ and CD8+ T cells; q value (FDR) and normalized enrichment score (NES) determined using fgsea package on R.
Extended Data Fig. 2 Analysis of published single-cell transcriptomic studies in UC cohorts.
a, Analysis of published single-cell datasets from UC cohorts4,5,6. UMAP showing Seurat-based clustering of colonic T cells, colored based on cluster type; the major T cell subsets (annotated by authors) present in each cluster are shown in Supplementary Table 5. UMAPs (left to right) show Seurat-normalized expression levels of genes enriched in the stem-like CD4+ T cells, stem-like CD8+ T cells, and CXCL13 or CXCR5 gene transcripts in single cells. Stem-like genes were identified in our dataset (Supplementary Table 4).
Extended Data Fig. 3 Stem-like T cells are enriched in inflamed regions.
a, Percentage of the CD4+ and CD8+ colonic T cell subsets from the indicated sources of colonic tissue samples obtained from each study subject. b, Plot shows Z-score average expression (color scale) and percentage of cells (size scale) that expressed the selected gene transcripts that were significantly enriched (FDR ≤ 0.05) in the indicated colonic CD8+ T cell clusters when compared to the other CD8+ T cells. c, UMAP shows Seurat-normalized expression levels of the indicated gene transcripts in single colonic CD8+ colonic T cells.
Extended Data Fig. 4 Expression of TFH and pro-inflammatory genes by colonic T cells in UC.
a, (Left) CD4, CD8, BCL-6 immunohistochemistry staining of colonic tissue samples. Indicated markers are light red, the CD31 marker staining, as described in methods, is in a dark brown color. (Right) BCL-6+CD4+ and BCL-6+CD8+ T cells counts from the indicated groups (n = 5 for healthy; n = 4 for inflamed tissue from patients with active UC). b, UMAPs show Seurat-normalized expression level of indicated gene transcripts in colonic CD4+ single cells. c, UMAP shows Seurat-normalized expression level of IL21R transcripts in colonic single CD4+ and CD8+ cells. Bar graphs in (a) depict mean ± SEM, each symbol represents data from an individual human subject. Statistical significance for the comparisons was computed using Student’s unpaired two-tailed t test (a).
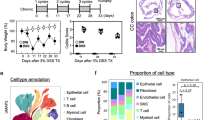
Extended Data Fig. 5 BCL-6-deficient mouse CD4+ T cells induced less colitis and had reduced TH17 cells.
a, Flow-cytometric plots show sequential gating strategy for isolating CD4+CD45RBhighCD25– T cells from spleen. b, Three experimental repeats of adoptive T cell transfer model of colitis in which 2.5 ×105 splenic CD4+CD45RBhighCD25– T cells from Bcl6fl/flCd4Cre mice or Bcl6fl/fl mice were transferred into Rag1−/− mice (numbers of mice in each experiment are shown in the figure); Body weight measurements of recipient Rag1−/− mice; plots depicts mean ± SEM. c, Flow-cytometric plots show sequential gating strategy for isolating CD4+ T cells from colon LP of recipient Rag1−/− mice and analyzing cells for intracellular cytokine production. d, Representative flow cytometry contour plots and frequency of colon LP CD4+ T cells that express the indicated cytokines following ex vivo stimulation with PMA plus ionomycin for 4 hours (Bcl6fl/fl n = 4; Bcl6fl/flCd4Cre n = 5). e, UMAPs show Seurat-normalized expression levels of Ccl5 and Gzmk gene transcripts in single colonic CD4+ T cells. f, Representative flow cytometry contour plots and frequency of CD4+ T cells that express IL-17A after in vitro differentiation under TH17 conditions (Bcl6fl/fl n = 3; Bcl6fl/flCd4Cre n = 3); experiment was performed once. Bar graphs in (d, f) depict mean ± SEM, each symbol represents data from an individual mouse; all data from are representative from one of at least two independent experiments with similar results. Statistical significance for the comparisons was computed using two-way ANOVA (b), Student’s unpaired two-tailed t test (d, f).
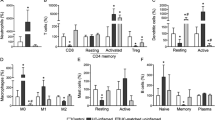
Extended Data Fig. 6 TCF1-deficiency attenuated pathogenicity of CD4+ T cells.
a, (Left) CD4, CD8, TCF1, and Hoechst immunofluorescence staining of colonic tissues. TCF1 detects proliferating epithelial cells as well as some T cells. Nuclear TCF1 staining in T cells is surrounded by a rim of CD4 or CD8 staining, as indicated by arrows for representative cells. (Right) TCF1+CD4+ and TCF1+CD8+ T cell counts from the indicated groups (n = 7 for healthy; n = 11 for inflamed tissue from patients with active UC). b, CD4+ T cell numbers from colon LP of the indicated recipient Rag1−/− mice (Tcf7fl/fl n = 6; Tcf7fl/flCd4Cre n = 6). c, Frequency of unstimulated colon LP CD4+ T cells that expressed surface CD8a or FOXP3+ and colon CD4+ T cells that expressed IFNg or IL-17A following ex vivo stimulation with PMA plus ionomycin for 4 hours (Tcf7fl/fl n = 7; Tcf7fl/flCd4Cre n = 6). d, Body weight measurements of recipient Rag1−/− mice (Tcf7fl/fl n = 6; Tcf7fl/flCd4Cre n = 6); the graph depicts mean ± SEM. Bar graphs in (a) depict mean ± SEM, each symbol represents data from an individual human subject. Bar graphs in (b-c) depict mean ± SEM, each symbol represents data from an individual mouse. Data (b-c) are representative from one of at least two independent experiments with similar results. Statistical significance for the comparisons was computed using Mann-Whitney two-sided test (a), Student’s unpaired two-tailed t test (b,c), two-way ANOVA (d).
Extended Data Fig. 7 TCF1 expressing stem-like mouse CD4+ T cells induced more colitis.
a, Flow-cytometric plots showing co-staining of intracellular GFP and TCF1 in the indicated mice. b, Flow-cytometric plots showing the frequency of GFP+CD4+ and GFP+CD8+ T cells in the spleen of Tcf7GFP reporter mice. c, GSEA plot shows enrichment of the indicated gene signatures in the GFP+CD4+ and GFP–CD4+ T cells in colon LP lymphocytes (LPL) of recipient Rag1−/− mice. d, Body weight measurements of the secondary recipient Rag1−/− mice (n = 4 or 6 for each group); the graph depicts mean ± SEM. e, Representative histology images in Fig. 7d. f, CD4+ T cell numbers from colon LP of the indicated recipient Rag1−/− mice (n = 4 or 6 for each group). Bar graphs in (f) depict mean ± SEM, each symbol represents data from an individual mouse. Data (d,e,f) are from one experiment. Statistical significance for the comparisons was computed using two-way ANOVA (d), Kruskal–Wallis test and adjustments were made for multiple comparisons (f).
Extended Data Fig. 8 Mouse colonic CD8+ T function was dependent of CD4+ T cells.
a, Flow-cytometric plots show sequential gating strategy used for isolating TCRβ+CD8β+CD4− T cells from spleen. b, Histology scores for the degree of colon tissue inflammation (n = 5 for CD4+ + CD8+ T cell transfer; n = 6 for CD8+ T cell transfer). c, Representative flow cytometry contour plots and frequency of colon LP CD8+ T cells that expressed the indicated cytokines following ex vivo stimulation with PMA-Ionomycin for 4 hours (n = 5 for CD4+ + CD8+ T cell transfer; n = 6 for CD8+ T cell transfer). Bar graphs in (b,c) depict mean ± SEM, each symbol represents data from an individual mouse; all data from are representative of one of two independent experiments with similar results except that histology in (b) was performed only once. Statistical significance for the comparisons was computed using one-way ANOVA (b), Student’s unpaired two-tailed t test (c).
Supplementary information
Source data
Source Data Fig. 1
GSEA plots source data.
Source Data Fig. 2
Statistical source data and gene expression source data.
Source Data Fig. 3
Statistical source data and gene expression source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data and GSEA plots source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data and GSEA plots source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Ramírez-Suástegui, C., Harris, R. et al. Stem-like T cells are associated with the pathogenesis of ulcerative colitis in humans. Nat Immunol 25, 1231–1244 (2024). https://doi.org/10.1038/s41590-024-01860-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41590-024-01860-7
This article is cited by
-
Correlation and diagnostic significance of CD4 T cell subsets and NLRP3 inflammasome in ulcerative colitis: the role of the NLRP3/T-bet/GATA3 axis
BMC Gastroenterology (2025)
-
Common mechanisms of Gut microbe-based strategies for the treatment of intestine-related diseases: based on multi-target interactions with the intestinal barrier
Cell Communication and Signaling (2025)
-
Tissue factor-dependent colitogenic CD4+ T cell thrombogenicity is regulated by activated protein C signalling
Nature Communications (2025)
-
Immune perturbations in human pancreas lymphatic tissues prior to and after type 1 diabetes onset
Nature Communications (2025)
-
RNA cytidine acetyltransferase NAT10 maintains T cell pathogenicity in inflammatory bowel disease
Cell Discovery (2025)