Abstract
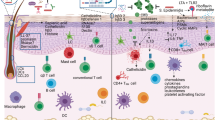
Human microbiome studies have revealed the intricate interplay of host immunity and bacterial communities to achieve homeostatic balance. Healthy skin microbial communities are dominated by bacteria with low viral representation1,2,3, mainly bacteriophage. Specific eukaryotic viruses have been implicated in both common and rare skin diseases, but cataloging skin viral communities has been limited. Alterations in host immunity provide an opportunity to expand our understanding of microbial–host interactions. Primary immunodeficient patients manifest with various viral, bacterial, fungal, and parasitic infections, including skin infections4. Dedicator of cytokinesis 8 (DOCK8) deficiency is a rare primary human immunodeficiency characterized by recurrent cutaneous and systemic infections, as well as atopy and cancer susceptibility5. DOCK8, encoding a guanine nucleotide exchange factor highly expressed in lymphocytes, regulates actin cytoskeleton, which is critical for migration through collagen-dense tissues such as skin6. Analyzing deep metagenomic sequencing data from DOCK8-deficient skin samples demonstrated a notable increase in eukaryotic viral representation and diversity compared with healthy volunteers. De novo assembly approaches identified hundreds of novel human papillomavirus genomes, illuminating microbial dark matter. Expansion of the skin virome in DOCK8-deficient patients underscores the importance of immune surveillance in controlling eukaryotic viral colonization and infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequencing data and genome assemblies for this study are linked to the NCBI BioProject ID PRJNA471898.
References
Oh, J. et al. Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64 (2014).
Hannigan, G. D. et al. The human skin double-stranded DNA virome: topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio 6, e01578-15 (2015).
Oh, J. et al. Temporal stability of the human skin microbiome. Cell 165, 854–866 (2016).
Picard, C. et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J. Clin. Immunol. 35, 696–726 (2015).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Zhang, Q. et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J. Exp. Med. 211, 2549–2566 (2014).
Paez-Espino, D. et al. Uncovering Earth’s virome. Nature 536, 425–430 (2016).
de la Cruz Pena, M. J. et al. Deciphering the human virome with single-virus genomics and metagenomics. Viruses 10, e113 (2018).
Reyes, A. et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 466, 334–338 (2010).
Shi, M. et al. The evolutionary history of vertebrate RNA viruses. Nature 556, 197–202 (2018).
Shi, M. et al. Redefining the invertebrate RNA virosphere. Nature 540, 539–543 (2016).
Foulongne, V. et al. Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE 7, e38499 (2012).
Schowalter, R. M., Pastrana, D. V., Pumphrey, K. A., Moyer, A. L. & Buck, C. B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 7, 509–515 (2010).
Chen, X., Anstey, A. V. & Bugert, J. J. Molluscum contagiosum virus infection. Lancet Infect. Dis. 13, 877–888 (2013).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012).
Jiang, X. et al. Skin infection generates non-migratory memory CD8 + T(RM) cells providing global skin immunity. Nature 483, 227–231 (2012).
Meisel, J. S. et al. Commensal microbiota modulate gene expression in the skin. Microbiome 6, 20 (2018).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Oh, J. et al. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 23, 2103–2114 (2013).
Byrd, A. L. et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 9, eaal4651 (2017).
Kowarsky, M. et al. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc. Natl Acad. Sci. USA 114, 9623–9628 (2017).
de Villiers, E. M., Fauquet, C., Broker, T. R., Bernard, H. U. & zur Hausen, H. Classification of papillomaviruses. Virology 324, 17–27 (2004).
Bernard, H. U. et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401, 70–79 (2010).
De Vlaminck, I. et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 155, 1178–1187 (2013).
Legoff, J. et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: new clues in enteric graft-versus-host disease. Nat. Med. 23, 1080–1085 (2017).
de Jong, S. J. et al. Epidermodysplasia verruciformis: inborn errors of immunity to human beta-papillomaviruses. Front. Microbiol. 9, 1222 (2018).
Reese, T. A. et al. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe 19, 713–719 (2016).
Roossinck, M. J. Move over, bacteria! Viruses make their mark as mutualistic microbial symbionts. J. Virol. 89, 6532–6535 (2015).
Kopylova, E., Noe, L. & Touzet, H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28, 3211–3217 (2012).
Fadrosh, D. W. et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2, 6 (2014).
Jo, J. H. et al. Diverse human skin fungal communities in children converge in adulthood. J. Invest. Dermatol. 136, 2356–2363 (2016).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370 (2013).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Nurk, S., Meleshko, D., Korobeynikov, A. & Pevzner, P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834 (2017).
Schmieder, R. & Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864 (2011).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
Van Doorslaer, K. et al. The papillomavirus episteme: a central resource for papillomavirus sequence data and analysis. Nucleic Acids Res. 41, D571–D578 (2013).
Ondov, B. D. et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 17, 132 (2016).
Jiang, P. & Singh, M. SPICi: a fast clustering algorithm for large biological networks. Bioinformatics 26, 1105–1111 (2010).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
McIntyre, C. L., Knowles, N. J. & Simmonds, P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 94, 1791–1806 (2013).
Simmonds, P. et al. Proposals for the classification of human rhinovirus species C into genotypically assigned types. J. Gen. Virol. 91, 2409–2419 (2010).
de Villiers, E. M. Cross-roads in the classification of papillomaviruses. Virology 445, 2–10 (2013).
Wheeler, D. L. et al. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 31, 28–33 (2003).
Byrd, A. L. et al. Clinical PathoScope: rapid alignment and filtration for accurate pathogen identification in clinical samples using unassembled sequencing data. BMC Bioinformatics 15, 262 (2014).
Croucher, N. J. et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 43, e15 (2015).
Lopez-Bueno, A., Parras-Molto, M., Lopez-Barrantes, O., Belda, S. & Alejo, A. Recombination events and variability among full-length genomes of co-circulating molluscum contagiosum virus subtypes 1 and 2. J. Gen. Virol. 98, 1073–1079 (2017).
Xiang, Y. & Moss, B. Molluscum contagiosum virus interleukin-18 (IL-18) binding protein is secreted as a full-length form that binds cell surface glycosaminoglycans through the C-terminal tail and a furin-cleaved form with only the IL-18 binding domain. J. Virol. 77, 2623–2630 (2003).
Yue, J. C. & Clayton, M. K. A similarity measure based on species proportions. Comm. Stat. – Theor. M. 34, 2123–2131 (2005).
Acknowledgements
This study utilized the high-performance computational capabilities of the NIH Biowulf Linux cluster (http://hpc.nih.gov). We thank M. Park, P. Thomas, A. Young, S. Phang, A. Pradhan, V. Pillai, J. Fekecs, NIH Patient Photography, and W.-I. Ng for underlying efforts, and the Segre and Kong laboratories for helpful discussions. We appreciate the participation of patients and their families. This work was supported by the National Human Genome Research Institute, National Institute of Allergy and Infectious Diseases, National Cancer Institute, and National Institute of Arthritis and Musculoskeletal and Skin Diseases Intramural Research Programs.
Author information
Authors and Affiliations
Consortia
Contributions
O.T., S.C., H.C.S., A.F.F., J.A.S., and H.H.K. contributed to the design and conception of the study. Sequencing was carried out by NISC. O.T., S.C., C.D., S.-Q.L.-L., and X.H. performed the experiments and analyses. O.T., J.A.S., and H.H.K. drafted the manuscript. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7
Supplementary Table 1
DOCK8-deficient patients’ metadata
Supplementary Table 2
Sequencing statistics for all DNA samples
Supplementary Table 3
Kingdom-level relative abundance by sample of healthy adults and children
Supplementary Table 4
Kingdom-level relative abundance by sample of DOCK8-deficient patients
Supplementary Table 5
Viral family taxonomic classifications of all DOCK8-deficient patients’ skin
Supplementary Table 6
Polyomavirus relative abundance by sample and by patient
Supplementary Table 7
Herpesviridae relative abundance by sample and by patient
Supplementary Table 8
Anelloviridae relative abundance by sample and by patient
Supplementary Table 9
Percent and number of DNA reads mapped to each of the reference databases and novel HPV genomes
Supplementary Table 10
Novel HPV genomes taxonomy
Supplementary Table 11
Number of human papillomavirus types detected on each patient
Supplementary Table 12
Changes in human papillomavirus communities in longitudinal samplings
Supplementary Table 13
Sequencing statistics for all RNA samples
Supplementary Table 14
Viral family-level relative abundance by sample from RNA sequencing data
Supplementary Table 15
Ribosomal RNA reads in all RNA samples
Supplementary Table 16
Assembly statistics for de novo assembly of DNA data by metaSpades, individual samples
Supplementary Table 17
Assembly statistics for de novo assembly of RNA data by rnaSpades, individual samples
Rights and permissions
About this article
Cite this article
Tirosh, O., Conlan, S., Deming, C. et al. Expanded skin virome in DOCK8-deficient patients. Nat Med 24, 1815–1821 (2018). https://doi.org/10.1038/s41591-018-0211-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-018-0211-7
This article is cited by
-
Omics-based decoding of molecular and metabolic crosstalk in the skin barrier ecosystem
Cell Death & Differentiation (2026)
-
The potential use of therapeutics and prophylactic mRNA vaccines in human papillomavirus (HPV)
Virology Journal (2024)
-
Characterization of multiple human papillomavirus types in the human vagina following ovarian hormonal stimulation
Virology Journal (2024)
-
Altered skin microbiome, inflammation, and JAK/STAT signaling in Southeast Asian ichthyosis patients
Human Genomics (2024)
-
The persistence and stabilization of auxiliary genes in the human skin virome
Virology Journal (2023)