Abstract
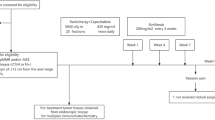
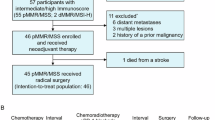
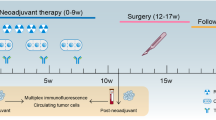
Radiotherapy displays unique antitumor synergism with immune checkpoint inhibitors, which is indicated by high pathological complete response (pCR) rates from single-arm trials of locally advanced rectal cancer (LARC). Here we test the efficacy and safety of the radiation–immune checkpoint inhibitor combination in patients with LARC in a phase 2, randomized trial conducted in eight major colorectal cancer centers in Beijing. In total, 186 eligible all-comer (proficient mismatch repair and deficient mismatch repair) participants were enrolled. The patients were randomly assigned to receive neoadjuvant chemoradiation + concurrent/sequential PD-1 blockade (experiment groups A/B) or neoadjuvant chemoradiation alone (control group). Radical surgeries were scheduled after neoadjuvant treatments. The primary endpoint was the pCR rate. The pCR rates were 27.1%, 32.7% and 14.0% for experiment groups A and B and the control group, respectively. The difference in pCR rates between experiment group B and the control group reached statistical significance (risk ratio 2.332, 95% confidence interval 1.106–4.916; P = 0.019). No substantial differences between either one of the experiment groups and the control group were observed regarding adverse reaction, surgical complication and disease progression. Our results show that adding PD-1 blockade after neoadjuvant chemoradiation increases the pCR rate for patients with LARC and raises no substantial safety concerns. Phase 3 trials with larger sample sizes are warranted (ClinicalTrials.gov identifier NCT05245474).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Deidentified individual-level patient data are shared as files in Supplementary Information, which contain the entirety of data for all-purpose validation of the results. The study protocol and the statistical analysis plan are also available as files in Supplementary Information. Source data are provided with this paper.
Code availability
Custom R codes used for the generation of forest plots are available at GitHub via https://github.com/pk2spl/Codes-of-the-POLARSTAR-trial/.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Wei, Q. et al. Incidence, prevention, risk factors, and prediction of venous thromboembolism in Chinese patients after colorectal cancer surgery: a prospective, multicenter cohort study. Int. J. Surg. 109, 3003–3012 (2023).
Cercek, A. et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376 (2022).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Ostwal, V. et al. Low prevalence of deficient mismatch repair (dMMR) protein in locally advanced rectal cancers (LARC) and treatment outcomes. J. Gastrointest. Oncol. 10, 19–29 (2019).
Ni, K. et al. Survival outcomes in locally advanced dMMR rectal cancer: surgery plus adjunctive treatment vs. surgery alone. BMC Cancer 23, 1013 (2023).
Kordbacheh, T., Honeychurch, J., Blackhall, F., Faivre-Finn, C. & Illidge, T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: building better translational research platforms. Ann. Oncol. 29, 301–310 (2018).
Lin, Z. et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J. Immunother. Cancer 9, e003554 (2021).
Shamseddine, A. et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat. Oncol. 15, 233 (2020).
Salvatore, L. et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): the AVANA study. Ann. Oncol. 39, 3511 (2021).
Bando, H. et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin. Cancer Res. 28, 1136–1146 (2022).
Gao, J. et al. Interim result of phase II, prospective, single-arm trial of long-course chemoradiotherapy combined with concurrent tislelizumab in locally advanced rectal cancer. Front. Oncol. 13, 1057947 (2023).
Yang, Z. et al. Efficacy and safety of PD-1 blockade plus long-course chemoradiotherapy in locally advanced rectal cancer (NECTAR): a multi-center phase 2 study. Signal Transduct. Target Ther. 9, 56 (2024).
Martin, S. T., Heneghan, H. M. & Winter, D. C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br. J. Surg. 99, 918–928 (2012).
Maas, M. et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 11, 835–844 (2010).
Galluzzi, L., Aryankalayil, M. J., Coleman, C. N. & Formenti, S. C. Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 20, 543–557 (2023).
Li, Z. et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv. Sci. 9, e2201734 (2022).
Lim, Y. J. et al. Chemoradiation-induced alteration of programmed death-ligand 1 and CD8+ tumor-infiltrating lymphocytes identified patients with poor prognosis in rectal cancer: a matched comparison analysis. Int. J. Radiat. Oncol. Biol. Phys. 99, 1216–1224 (2017).
Shinto, E. et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann. Surg. Oncol. 21, S414–S421 (2014).
Garcia-Aguilar, J. et al. Timing of rectal cancer response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 16, 957–966 (2015).
Jin, J. et al. Multicenter, randomized, phase III trial of Short-Term Radiotherapy Plus Chemotherapy versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 40, 1681–1692 (2022).
Rahma, O. E. et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. 7, 1225–1230 (2021).
He, X. et al. Upfront dose-reduced chemotherapy synergizes with immunotherapy to optimize chemoimmunotherapy in squamous cell lung carcinoma. J. Immunother. Cancer 8, e000807 (2020).
Halmos, B. et al. A matching-adjusted indirect comparison of pembrolizumab + chemotherapy vs. nivolumab + ipilimumab as first-line therapies in patients with PD-L1 TPS ≥1% metastatic NSCLC. Cancers 12, 3648 (2020).
Swami, U. et al. Exceptional responses with sequential metronomic temozolomide after pembrolizumab failure in patients with metastatic melanoma. Melanoma Res. 29, 643–647 (2019).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Spigel, D. R. et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 40, 1301–1311 (2022).
Terzi, C. et al. Randomized controlled trial of 8 weeks’ vs 12 weeks’ interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer. Colorectal Dis. 22, 279–288 (2020).
Akgun, E. et al. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br. J. Surg. 105, 1417–1425 (2018).
Lefevre, J. H. et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J. Clin. Oncol. 34, 3773–3780 (2016).
Pang, K. BioRender. BioRender http://www.BioRender.com/b53j971 (2021).
Morton, D. et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J. Clin. Oncol. 41, 1541–1552 (2023).
Quirke, P., Durdey, P., Dixon, M. F. & Williams, N. S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Lancet 2, 996–999 (1986).
Trakarnsanga, A. et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J. Natl Cancer Inst. 106, dju248 (2014).
Fokas, E. et al. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomized phase III trial. Ann. Oncol. 29, 1521–1527 (2018).
Pang, K. et al. Long-course chemoradiation plus concurrent/sequential PD-1 blockade as neoadjuvant treatment for MMR-status-unscreened locally advanced rectal cancer: protocol of a multicentre, phase 2, randomised controlled trial (the POLAR-STAR trial). BMJ Open 13, e069499 (2023).
Acknowledgements
This trial is financially supported by Beijing Li Huanying Medical Foundation (to Z.Z.), Beijing Postdoctoral Research Foundation (grant 2023-ZZ-038, to K.P.), BeiGene (to Z.Z.) and National Key Technologies R&D Program (grant 2015BAI13B09, to Z.Z.). The experimental drugs (tislelizumab) are supplied by BeiGene. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.P. Data curation: J.G., J.Z., L.X., Z.G., Y.W., A.L., J.H., G.W., X.W., F.L., Y.Y., J.Z. and G.C. Formal analysis: K.P. and Y.K. Funding acquisition: K.P. and Z.Z. Investigation: Y.Y., K.P., G.L. and X.L. Methodology: Y.K., H.W., K.P., G.C. and J.Z. Project administration: Y.Y. Resources: A.L., J.H., G.W., X.W., Y.Y., A.W., Y.X. and Z.Z. Software: K.P. Supervision: Z.Z. Validation: H.Y. Visualization: K.P. Writing (original draft): K.P. Writing (review and editing): K.P., Y.Y., G.L., X.L., Y.K., A.W., Y.X., H.Y. and Z.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Robert Glynne-Jones, Xueliang Pan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jean Nakhle and Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Radiological tumor regression of the three groups.
MRI-recorded tumor regression of the primary lesion from baseline (i.e. pre-radiotherapy) to post-treatment (i.e. pre-surgery) re-evaluation.
Extended Data Fig. 2 Detailed distribution of the NAR scores in the three groups.
Concurrent plan: n = 57, sequential plan: n = 51, control: n = 46. (A) The distribution of the NAR scores along with the best fit normal distribution curve in the three groups. Red arrow points to the column of patients with low NAR score. (B) Comparison of NAR scores with boxplot. Data are presented as median values (centres) and interquartile range (left and right boundaries of boxes), with whiskers indicating 5-95 percentiles and dots indicating outlier values.
Extended Data Fig. 3 Subgroup analysis of pCR rate.
Data are presented with forest plot, with centres indicating values of risk ratios (RR) and error bars indicating 95% confidence intervals of RRs. (A) between Experiment group A and Control group. (B) between Experiment group B and Control group.
Supplementary information
Supplementary Information
Trial protocol and statistical analysis plan.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Table 1
Statistical source data.
Source Data Table 2
Statistical source data.
Source Data Table 3
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Table 1
Statistical source data.
Source Data Extended Data Table 4
Statistical source data.
Source Data Extended Data Table 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Pang, K., Lin, G. et al. Neoadjuvant chemoradiation with or without PD-1 blockade in locally advanced rectal cancer: a randomized phase 2 trial. Nat Med 31, 449–456 (2025). https://doi.org/10.1038/s41591-024-03360-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03360-5
This article is cited by
-
The molecular mechanisms of pyroptosis and its implications in tumor immunotherapy
Molecular Cancer (2026)
-
Neoadjuvant chemoradiotherapy with or without PD-1/PD-L1 inhibitors in locally advanced rectal cancer: a systematic review and meta-analysis
BMC Cancer (2025)
-
Neoadjuvant chemoradiotherapy plus sintilimab in pMMR/MSS rectal cancer patients with PD-L1 TPS ≥ 1% or CPS ≥ 1: an open-label, prospective, phase II study
npj Precision Oncology (2025)
-
Development of the rationale of a personalized cancer vaccine based on the in situ vaccine effect of radiotherapy: a mechanistic study of the POLARSTAR trial
Cancer Immunology, Immunotherapy (2025)
-
Efficacy and safety of neoadjuvant therapy combined with immunotherapy in MMR‑proficient/microsatellite stable non‑metastatic rectal cancer: a systematic review and meta‑analysis
Journal of Cancer Research and Clinical Oncology (2025)