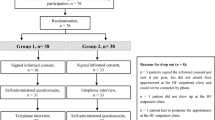
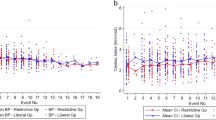
Abstract
Fluid restriction is frequently recommended to patients with chronic heart failure, but randomized clinical trials assessing the effects of fluid restriction remain scarce. In this multicenter open-label trial, outpatients with chronic heart failure were randomized to receiving advice for liberal fluid intake versus receiving advice for fluid restriction, up to 1,500 ml per day of fluid intake. The primary outcome of the trial was health status after 3 months, as assessed by the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS). Secondary outcomes included thirst distress and safety events. Among 504 randomized patients (67.3% male), the KCCQ-OSS after 3 months was 74.0 in the liberal fluid intake group versus 72.2 in the fluid restriction group, with a mean difference after adjustment for baseline scores of 2.17 (95% confidence interval −0.06 to 4.39; P = 0.06), indicating that the primary outcome was not met. Thirst distress was higher in the fluid restriction group and no differences were observed for safety events between the two groups. These findings question the benefit of fluid restriction in chronic heart failure. ClinicalTrials.gov registration: NCT04551729.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Anonymized participant data can be made available upon requests directed to the corresponding author. Data requests will be reviewed on the basis of scientific merit, ethical review, available resources and regulatory requirements, and a response can be expected within two weeks. Following approvals, unidentified patient data and a data dictionary will be provided. The steering committee will have the right to review and comment on any draft papers based on these data before publication.
References
Eng, S. H. et al. Thirst and factors associated with frequent thirst in patients with heart failure in Spain. Heart Lung 50, 86–91 (2021).
van der Wal, M. H. L., Waldreus, N., Jaarsma, T. & Kato, N. P. Thirst in patients with heart failure in Sweden, the Netherlands, and Japan. J. Cardiovasc. Nurs. 35, 19–25 (2020).
van der Wal, M. H. L., Jaarsma, T., Jenneboer, L. C. & Linssen, G. C. M. Thirst in stable heart failure patients; time to reconsider fluid restriction and prescribed diuretics. ESC Heart Fail. 9, 2181–2188 (2022).
Vazir, A. et al. Decongestion strategies in patients presenting with acutely decompensated heart failure: a worldwide survey among physicians. Eur. J. Heart Fail. 25, 1555–1570 (2023).
Hoog, L., Stromberg, A., Waldreus, N. & Nymark, C. A national survey of healthcare professional’s clinical practice on fluid intake in patients with heart failure. Abstract. Eur. J. Heart Fail. 26 (S2), 3–643 (2024).
De Vecchis, R., Baldi, C., Cioppa, C., Giasi, A. & Fusco, A. Effects of limiting fluid intake on clinical and laboratory outcomes in patients with heart failure. Results of a meta-analysis of randomized controlled trials. Herz 41, 63–75 (2016).
Li, Y., Fu, B. & Qian, X. Liberal versus restricted fluid administration in heart failure patients. A systematic review and meta-analysis of randomized trials. Int. Heart J. 56, 192–195 (2015).
Albert, N. M., Nutter, B., Forney, J., Slifcak, E. & Tang, W. H. A randomized controlled pilot study of outcomes of strict allowance of fluid therapy in hyponatremic heart failure (SALT-HF). J. Card. Fail. 19, 1–9 (2013).
Holst, M., Stromberg, A., Lindholm, M. & Willenheimer, R. Liberal versus restricted fluid prescription in stabilised patients with chronic heart failure: result of a randomised cross-over study of the effects on health-related quality of life, physical capacity, thirst and morbidity. Scand. Cardiovasc. J. 42, 316–322 (2008).
Reilly, C. M., Higgins, M. & Dunbar, S. B. Clinical, symptom, functional, and QOL outcomes in a trial of prescribed fluid restrictions in persons with heart failure. Circulation 136, A19846 (2017).
Travers, B. et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J. Card. Fail. 13, 128–132 (2007).
Holst, M., Stromberg, A., Lindholm, M. & Willenheimer, R. Description of self-reported fluid intake and its effects on body weight, symptoms, quality of life and physical capacity in patients with stable chronic heart failure. J. Clin. Nurs. 17, 2318–2326 (2008).
Waldreus, N., Hahn, R. G. & Jaarsma, T. Thirst in heart failure: a systematic literature review. Eur. J. Heart Fail. 15, 141–149 (2013).
Waldreus, N., Chung, M. L., van der Wal, M. H. & Jaarsma, T. Trajectory of thirst intensity and distress from admission to 4-weeks follow up at home in patients with heart failure. Patient Prefer. Adherence 12, 2223–2231 (2018).
van der Wal, M. H. et al. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur. Heart J. 27, 434–440 (2006).
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 79, e263–e421 (2022).
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726 (2021).
Mullens, W. et al. Dietary sodium and fluid intake in heart failure. A clinical consensus statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 26, 730–741 (2024).
Spertus, J. A., Jones, P. G., Sandhu, A. T. & Arnold, S. V. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 76, 2379–2390 (2020).
Abdel Jawad, M. et al. Interpreting population mean treatment effects in the Kansas City Cardiomyopathy Questionnaire: a patient-level meta-analysis. JAMA Cardiol. 10, 32–40 (2025).
Merlo, A., Mezue, K. & Ambrosy, A. P. Fluid restriction recommendations in heart failure: dry as a bone or quench your thirst? J. Card. Fail. 28, 1531–1533 (2022).
Herrmann, J. J. et al. Fluid REStriction in heart failure vs liberal fluid UPtake: rationale and design of the randomized FRESH-UP study. J. Card. Fail. 28, 1522–1530 (2022).
Waldreus, N., Jaarsma, T., van der Wal, M. H. & Kato, N. P. Development and psychometric evaluation of the Thirst Distress Scale for patients with heart failure. Eur. J. Cardiovasc. Nurs. 17, 226–234 (2018).
Green, C. P., Porter, C. B., Bresnahan, D. R. & Spertus, J. A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J. Am. Coll. Cardiol. 35, 1245–1255 (2000).
Eng, S. H. et al. Thirst distress in outpatients with heart failure in a Mediterranean zone of Spain. ESC Heart Fail. 8, 2492–2501 (2021).
McMurray, J. J. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Veazie, P. J. et al. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J. Am. Coll. Cardiol. 60, 1940–1944 (2012).
Lewis, E. F. et al. Health-related quality of life outcomes in PARADIGM-HF. Circ. Heart Fail. 10, e003430 (2017).
Ekman, I. et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur. Heart J. 32, 2395–2404 (2011).
Acknowledgements
We thank the Netherlands Heart Institute for their logistical support, and both the Dutch Heart Foundation (grant no. 2019T100 to R.R.J.v.K.) as well as the Academic Alliance Fund of the Radboud University Medical Center, Nijmegen and Maastricht University Medical Center (grant R0005106) for their financial support (H.-P.B.-L.R., F.B.-W., D.H.F.G., R.R.J.v.K.). The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Author information
Authors and Affiliations
Contributions
H.-P.B.-L.R., T.J., F.B.-W., L.B., D.H.F.G. and R.R.J.v.K. wrote the study protocol. All authors approved the protocol. L.E.H.J.M.B., F.B.-W., M.H.I.V., D.H.F.G. and R.R.J.v.K. acquired funding for the project. L.R. and D.H.F.G. planned the statistical analysis, which was executed by J.J.H. and L.R. J.J.H. and L.R. accessed and verified the underlying study data. J.J.H., H.-P.B.-L.R., L.E.H.J.M.B., F.B.-W., S.C.A.M.B., L.B., J.W.M.v.E., H.C.H., G.C.M.L., R.P., S.S.-v.W. and M.H.I.V. participated in patient enrollment and carried out the trial. J.J.H. drafted the initial version of the manuscript with input from D.H.F.G. and R.R.J.v.K. All authors reviewed the data analyses, data interpretation and writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors have seen and approved of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.-P.B.-L.R. has received research grants from Innovative Health Initiative Joint Undertaking and Roche Diagnostics, received consulting fees from Astra Zeneca, Boehringer Ingelheim, Novartis, Roche Diagnostics and Vifor Pharma, received honoraria from Roche Diagnostics and participated on an Advisory Board of CeleCor Therapeutics. S.S.-v.W. has received research grants from Boehringer Ingelheim, CRL funds and ZonMW, received honoraria from Astra Zeneca, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, Roche Diagnostics and Pfizer, and is a member of the board of the Werkgroep Cardiologisch Centra Nederland and Stichting Perfusie. P.v.d.M. has received research grants from Astra Zeneca, Pfizer, Pharma Nord, Novo Nordisk, Ionis and Vifor Pharma, honoraria from Astra Zeneca, Boehringer Ingelheim, BridgeBio, Daiichi Sankyo, Ionis, Novartis, Novo Nordisk, Pfizer, Pharma Nord, Pharmacosmos and Vifor Pharma, and participated on an Advisory Board for CorHeart. J.L.J. has received research grants from Abbott, Applied Therapeutics, Astra Zeneca, HeartFlow and Novartis, received consulting fees from Abbott, Applied Therapeutics, Astra Zeneca, Beckman, Boehringer Ingelheim, Bristol Myers, Intellia, Jana Care, Novartis, Pfizer, Merck, Roche Diagnostics and Siemens, participated on an Advisory Board for Abbott, AbbVie, Bayer, CVRx, Roche Diagnostics and Takeda, is a member of the board of American College of Cardiology and Imbria Pharmaceuticals, and has stock (options) in Imbria Pharmaceuticals and Jana Care. A.B.-G. has lectured and/or participated in advisory boards for Abbott, Astra Zeneca, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics and Vifor Pharma. D.H.F.G. received a speaker’s fee from Novartis and travel support from Astra Zeneca. R.R.J.v.K. received consulting fees from Novo Nordisk and Roche Diagnostics, honoraria from Hippocrates Academy and is a fiduciary member of the ESC working group on Peripheral Artery and Aortic Disease. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Justin Ezekowitz, Wilfried Mullens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Win ratio.
The win ratio for safety outcomes during the six months follow-up.
Extended Data Fig. 2 Primary outcome, according to pre-specified subgroup: study site.
The primary outcome of the trial, adjusted mean difference in the KCCQ Overall Summary, tested two-sided with ANCOVA analysis using baseline KCCQ Overall Summary Score as covariate, according to the pre-specified subgroup: study site.
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary Information (steering committee members, data safety and monitoring board members, independent event adjudication committee members, principal investigators; inclusion and exclusion criteria; standardized lifestyle advice and Thirst Distress Scale for patients with heart failure), study protocol, summary of changes and statistical analysis plan.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herrmann, J.J., Brunner-La Rocca, HP., Baltussen, L.E.H.J.M. et al. Liberal fluid intake versus fluid restriction in chronic heart failure: a randomized clinical trial. Nat Med 31, 2062–2068 (2025). https://doi.org/10.1038/s41591-025-03628-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03628-4
This article is cited by
-
Hyponatraemia in heart failure: a mechanistic approach to contemporary management
Heart Failure Reviews (2026)
-
Let the drinks flow in chronic heart failure? Insights from the FRESH-UP study
Heart Failure Reviews (2025)
-
Update on clinical heart failure trials
Clinical Research in Cardiology (2025)
-
Therapie der Herzinsuffizienz nach Dekompensation
MMW - Fortschritte der Medizin (2025)