Abstract
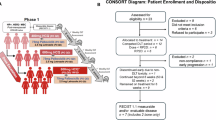
Breast cancer recurrence may arise from dormant disseminated tumor cells (DTCs) that persist in bone marrow and other sites. Clinically, DTCs are independently associated with breast cancer recurrence and death. Preclinical studies in mouse models identified autophagy and mammalian target of rapamycin (mTOR) signaling as critical mechanisms of tumor dormancy and escape. We subsequently tested the effects of transient versus chronic inhibition of autophagy with chloroquine or hydroxychloroquine (HCQ) and mTOR signaling with rapamycin (RAPA) or everolimus (EVE) on residual tumor cell (RTC) burden and recurrence-free survival (RFS). In mice harboring dormant RTCs, inhibition of mTOR alone or in combination with autophagy inhibition decreased RTC burden and improved RFS in a duration-dependent manner. RTC number was strongly and inversely correlated with RFS, suggesting that RTC reduction mediated an improvement in RFS. To translate findings clinically, we performed a randomized phase 2 trial (CLEVER) of HCQ, EVE or their combination in breast cancer survivors within 5 years of diagnosis who had detectable DTCs on bone marrow aspirate. Primary endpoints were feasibility and safety; secondary endpoints included DTC reduction/clearance and RFS. In total, 51 DTC+ patients initiated HCQ (n = 15), EVE (n = 15) or HCQ + EVE (n = 21). Treatment was feasible and tolerable; only one patient discontinued early for grade 3 toxicity. At 42 months median follow-up, landmark 3-year RFS for HCQ, EVE and HCQ + EVE was 91.7%, 92.9% and 100%, respectively, and was greater in those who cleared DTCs versus those who did not (hazard ratio (HR) = 0.21 (95% confidence interval 0.01–3.4)). Posterior probabilities were 98–99.9% that three cycles of HCQ, EVE or HCQ + EVE led to reduced or undetectable DTCs compared to observation alone, with estimated DTC reductions of 80%, 78% and 87%, respectively. These findings provide proof-of-concept that targeting dormant RTCs with HCQ, EVE or their combination in breast cancer survivors or mouse models depletes minimal residual disease, warranting a definitive human randomized controlled trial. ClinicalTrials.gov registration: NCT03032406.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper. The data used and/or analyzed during the current study are available in the Supplementary Information or from the corresponding author(s) on request, recognizing that certain patient-related data not included in the paper were generated as part of the clinical trial and may be subject to patient confidentiality. It is estimated that the corresponding authors will respond to external data requests within 2 weeks of receipt of the request to verify whether the request is subject to any intellectual property or confidentiality obligations. Uncropped original western blots corresponding to Extended Data Fig. 2e,f,k,l are provided. The authors do not have IRB approval or patient consent to share identifying or sensitive data on CLEVER clinical trial participants and therefore cannot report data in a public repository. Source data are provided with this paper.
Code availability
This study used custom code for Bayesian data modeling, which will be made available upon request. It is estimated that the corresponding authors will respond to requests for code within 2 weeks of receipt of the request.
References
Pedersen, R. N. et al. The incidence of breast cancer recurrence 10–32 years after primary diagnosis. J. Natl Cancer Inst. 114, 391–399 (2022).
Colleoni, M. et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J. Clin. Oncol. 34, 927–935 (2016).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 19, 27–39 (2018).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005).
Loi, S., Buyse, M., Sotiriou, C. & Cardoso, F. Challenges in breast cancer clinical trial design in the postgenomic era. Curr. Opin. Oncol. 16, 536–541 (2004).
Cescon, D. W. et al. Therapeutic targeting of minimal residual disease to prevent late recurrence in hormone-receptor positive breast cancer: challenges and new approaches. Front. Oncol. 11, 667397 (2021).
Banys-Paluchowski, M., Reinhardt, F. & Fehm, T. Disseminated tumor cells and dormancy in breast cancer progression. Adv. Exp. Med. Biol. 1220, 35–43 (2020).
Roy, R. et al. Escape from breast tumor dormancy: the convergence of obesity and menopause. Proc. Natl Acad. Sci. USA 119, e2204758119 (2022).
Ruth, J. R. et al. Cellular dormancy in minimal residual disease following targeted therapy. Breast Cancer Res. 23, 63 (2021).
Ecker, B. L. et al. Impact of obesity on breast cancer recurrence and minimal residual disease. Breast Cancer Res. 21, 41 (2019).
Abravanel, D. L. et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J. Clin. Invest. 125, 2484–2496 (2015).
Dalla, E., Sreekumar, A., Aguirre-Ghiso, J. A. & Chodosh, L. A. Dormancy in breast cancer. Cold Spring Harb. Perspect. Med. 13, a041331 (2023).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Hall, C. et al. Disseminated tumor cells predict survival after neoadjuvant therapy in primary breast cancer. Cancer 118, 342–348 (2012).
Mathiesen, R. R. et al. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res. 14, R117 (2012).
Hartkopf, A. D. et al. Disseminated tumour cells from the bone marrow of early breast cancer patients: results from an international pooled analysis. Eur. J. Cancer 154, 128–137 (2021).
Fehm, T. et al. Pooled analysis of the prognostic relevance of disseminated tumor cells in the bone marrow of patients with ovarian cancer. Int. J. Gynecol. Cancer 23, 839–845 (2013).
Moody, S. E. et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2, 451–461 (2002).
Gunther, E. J. et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 17, 488–501 (2003).
Moody, S. E. et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 (2005).
Vera-Ramirez, L., Vodnala, S. K., Nini, R., Hunter, K. W. & Green, J. E. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 9, 1944 (2018).
Dwyer, S., Ruth, J., Seidel, H. E., Raz, A. A. & Chodosh, L. A. Autophagy is required for mammary tumor recurrence by promoting dormant tumor cell survival following therapy. Breast Cancer Res. 26, 143 (2024).
Paul, M. R. et al. Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J. Clin. Invest. 130, 4252–4265 (2020).
D’Cruz, C. M. et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat. Med. 7, 235–239 (2001).
Sreekumar, A. et al. B3GALT6 promotes dormant breast cancer cell survival and recurrence by enabling heparan sulfate-mediated FGF signaling. Cancer Cell 42, 52–69 (2024).
Finbloom, D. S., Silver, K., Newsome, D. A. & Gunkel, R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J. Rheumatol. 12, 692–694 (1985).
La Belle Flynn, A. et al. Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nat. Commun. 10, 3668 (2019).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Aqbi, H. F. et al. Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy. Oncotarget 9, 22113–22122 (2018).
Lu, Z. et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Invest. 118, 3917–3929 (2008).
Chery, L. et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5, 9939–9951 (2014).
Marshall, J. C. et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J. Natl Cancer Inst. 104, 1306–1319 (2012).
Kobayashi, A. et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 208, 2641–2655 (2011).
Bragado, P. et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol. 15, 1351–1361 (2013).
Feng, Y. et al. SPSB1 promotes breast cancer recurrence by potentiating c-MET signaling. Cancer Discov. 4, 790–803 (2014).
Alvarez, J. V. et al. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 24, 30–44 (2013).
Chen, S. et al. PAQR8 promotes breast cancer recurrence and confers resistance to multiple therapies. Breast Cancer Res. 25, 1 (2023).
Rueda, O. M. et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 567, 399–404 (2019).
Bidard, F. C. et al. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin. Cancer Res. 14, 3306–3311 (2008).
Naume, B. et al. Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol. 32, 3848–3857 (2014).
Consortium, I. S. T. et al. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 6, 1355–1362 (2020).
Chavez-MacGregor, M. et al. Phase III randomized, placebo-controlled trial of endocrine therapy ± 1 year of everolimus in patients with high-risk, hormone receptor-positive, early-stage breast cancer. J. Clin. Oncol. 42, 3012–3021 (2024).
Coombes, R. C. et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin. Cancer Res. 25, 4255–4263 (2019).
Garcia-Murillas, I. et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 7, 302ra133 (2015).
Kaplan, E. L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481 (1958).
Tukey, J. W. Exploratory Data Analysis (Addison-Wesley, 1977).
Mann, H. B. & Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 18, 50–60 (1947).
Fehm, T. et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 107, 885–892 (2006).
Tolaney, S. M. et al. Updated standardized definitions for efficacy end points (STEEP) in adjuvant breast cancer clinical trials: STEEP version 2.0. J. Clin. Oncol. 39, 2720–2731 (2021).
Acknowledgements
We thank the Abramson Cancer Center and Penn Medicine for their support of the 2-PREVENT Translational Center of Excellence, as well as the staff and members of the 2-PREVENT TCE for their efforts in support of the CLEVER study. We acknowledge support from the following funders: the National Cancer Institute (R01CA208273 to A.D. and L.C.), Department of Defense (BC160784 to A.D. and L.C.), V Foundation, Breast Cancer Research Foundation (to A.D. and L.C.), QVC ‘Shoes on Sale’ (to A.D.), Jerry S. Rosenbloom (to A.D. and L.C.), Sara and Jim Gowing (to A.D. and L.C.), Avon Foundation (to A.D. and L.C.), Raynier Institute & Foundation (to A.D. and L.C.), Rhoda Polly Danziger and Michael Danziger (to L.C.), Andrea Orsher and Robert Orsher (to A.D.), the Dietz & Watson Family (to A.D.), and Novartis for providing Everolimus for the CLEVER trial. We appreciate the important contribution of the 2-PREVENT TCE Patient Advocate Board—J. Perlmutter, J. LaScala, C. Abi-Khattar, J. Bowles, M. Ayres, L. Mikulski and S. Axler. Finally, we are indebted to the CLEVER trial participants and their families, without whom this research would not be possible. This study was presented at the European Society of Clinical Oncology on 23 October 2023.
Author information
Authors and Affiliations
Contributions
L.A.C. and A.D. conceived of the approach to prevent recurrence by depleting MRD, designed the overall study, oversaw its conduct and obtained funding to support it. A.D. wrote the CLEVER protocol and provided oversight of study conduct. A.D., A.S.C. and J.S. provided clinical care to CLEVER trial participants and charted source documentation of clinical research visits. A.D., A.S.C. and J.S. conducted study visits and clinical management of study patients. L.J.B. provided project management, supervision of staff, input on regulatory matters and design of case report forms. K.R. cleaned and prepared CLEVER data for analysis. K.R., L.R.B., D.B., L.J.B., L.A.C. and A.D. generated figures (Figs. 2 and 3 and Extended Data Fig. 4) describing CLEVER trial results. I.N. coordinated participant visits and data collection on the CLEVER trial and assisted with patient sample collection. P.W. analyzed CLEVER feasibility data. L.R.B. and D.B. analyzed and interpreted CLEVER recurrence-free survival and DTC-IHC data. S.D. collected and tracked patient samples and managed sample inventory. S.E.D. and L.A.C. generated, analyzed and interpreted mouse preclinical data on the effects of CQ and RAPA on RTC number and recurrence-free survival, on which the mouse study and CLEVER trial were based. C.J.S., N.M., G.K.B. and S.E.D. performed mouse studies. Y.C. and A.E. processed mouse tumor samples and performed ddPCR to enumerate RTCs. E.S., T.C.P., D.K.P., G.K.B. and L.A.C. analyzed and interpreted mouse CLEVER recurrence-free survival and residual disease data. H.M. and E.S. performed and quantified western blots on mouse samples. E.S., J.W. and G.K.B. performed and analyzed mouse immunofluorescence studies. E.S., T.C.P., G.K.B. and L.A.C. generated figures describing mouse preclinical study results. G.K.B. and L.A.C. provided project management. G.K.B. provided supervision, wrote animal protocols and provided input on regulatory matters. B.L.G. performed patient BMAs. J.W., E.M.C. and J.G. contributed to the processing of patient samples. E.M.C. and J.G. provided control cell lines for the DTC-IHC assay, assisted in transitioning the DTC-IHC assay to a Clinical Laboratory Improvement Amendments (CLIA) laboratory and performed hemodilution assessment of BMAs. E.M.C., N.S., L.J.B. and I.M. contributed to the design and reporting of DTC-IHC assay rescreen testing and the implementation of the dual readers and adjudication system. M.F. and N.S. oversaw the transition of the DTC-IHC assay to a CLIA laboratory. M.F. and A.N. evaluated patient bone marrow samples for the presence of DTCs by DTC-IHC, adjudicated discordant reads and generated source pathology reports documenting DTC-IHC results. N.S., L.J.B. and S.D. provided operational support and coordination for sample collection and processing for the DTC-IHC assay. A.D., L.A.C. and E.S. drafted the manuscript. All authors approved the final manuscript and contributed to critical revisions of its intellectual content.
Corresponding authors
Ethics declarations
Competing interests
A.D. has received institutional research funding from Novartis, Genentech, Pfizer and NeoGenomics. L.A.C. has received institutional research funding from Novartis, AstraZeneca and Merck Research Laboratories, and has served as an expert consultant to Teva Pharmaceuticals, Eisai, Sanofi, Takeda Pharmaceuticals, Eli Lilly, Whittaker, Clark and Daniels, Wyeth, Imerys, Becton Dickinson, Sterigenics and the U.S. Department of Justice in litigation. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: J. Nakhle and S. Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presentation of the study European Society of Clinical Oncology, 23 October 2023.
Extended data
Extended Data Fig. 1 Recurrence-free survival outcomes in mouse CLEVER preclinical study following chronic treatment with study agents.
a, Schematic of orthotopic inducible Her2 mouse model and timing of rapamycin treatment in mice bearing dormant residual tumor cells. b, Recurrence-free survival of female nu/nu mice harboring dormant residual disease from fully regressed primary tumors treated with vehicle control (VEH) or 4 mg/kg RAPA (n = 20) initiated 28 d after Her2 downregulation. c–f, RFS outcomes for mice treated chronically with VEH, RAPA, EVE, CQ, HCQ or EVE + HCQ. c, RAPA or CQ compared to vehicle control (VEH); d, EVE or HCQ compared to VEH; e, RAPA or EVE compared to VEH; or f, HCQ or CQ compared to VEH. RFS outcomes shown in panels c–f and in Fig. 1 represent a single study with 16 treatment arms, where the results for different comparisons between treatment arms and treatment durations are shown as individual panels for clarity. Hazard ratios (HR) and unadjusted p values from the two-sided log-rank test are shown for the indicated comparisons.
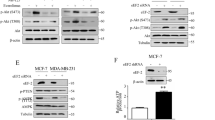
Extended Data Fig. 2 Pharmacodynamic analysis of study agents.
a–d, Quantification of western blots for phospho-S6 (a,b) or LC3-II (c,d) performed on lysates of proliferative (a,c) or dormant (b,d) Her2-inducible primary tumor cells in vitro treated with vehicle (VEH), rapamycin (RAPA), everolimus (EVE), hydroxychloroquine [HCQ] or EVE + HCQ. A recurrent Her2 tumor cell line is shown as a control for proliferative samples. Proliferative and dormant VEH controls are shown for scale to the right. Representative western blots for proliferative (e) and dormant (f) tumor cells corresponding to a–d. g–j, Quantification of western blots for phospho-S6 (a,b) or LC3-II (c,d) performed on lysates of primary orthotopic tumors (g,i) or dormant residual orthotopic tumors (h,j) derived from Her2-inducible primary tumor cells collected from mice treated with VEH, RAPA, EVE, CQ, HCQ or EVE + HCQ. Primary tumor and dormant tumor VEH controls are shown for scale to the right. Representative western blots for primary tumor (k) and dormant (l) tumor lysates corresponding to g–j. Bars and error bars show mean ± s.e.m. Molecular weight markers are shown. For l, a dotted vertical line indicates where the molecular weight marker lanes were not immediately adjacent to the samples shown (which were otherwise contiguous).
Extended Data Fig. 3 Impact of treatment on residual tumor cell number in mouse CLEVER preclinical study.
a, Relative change in residual tumor cell number (RTC) count following 3 weeks or 9 weeks of treatment with the indicated therapies, shown as % change relative to vehicle control. b, Absolute RTC counts following 3 weeks or 9 weeks of treatment with the indicated therapies or vehicle control. Red boxes indicate residual tumor lesions that were classified as recurrent tumors based on progressive increases in tumor size. Purple triangles indicate RTC counts that were statistical outliers. MGRD, mammary gland residual disease. c, Plot of median RFS versus log10 median RTC counts following 3 weeks or 9 weeks of treatment with the indicated therapies or vehicle control shown as a combined cohort. The Pearson correlation coefficient is shown for the combined cohort and tested against the t distribution for estimating a two-sided p value.
Extended Data Fig. 4 Recurrence-free survival by receptor status and DTC status in the CLEVER trial.
a, Recurrence-free survival by receptor status. To avoid overlapping RFS curves, a small amount of vertical spacing has been added to the lines at 100% RFS to distinguish between the different receptor groups. b, Recurrence-free survival by DTC detection status ascertained after cycle 6 of treatment. Number of patients at risk at each time point is shown.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Source data
Source Data Extended Data Fig. 2, panel e
Unprocessed western blots
Source Data Extended Data Fig. 2, panel e
Unprocessed western blots
Source Data Extended Data Fig. 2, panel e
Unprocessed western blots
Source Data Extended Data Fig. 2, panel e
Unprocessed western blots
Source Data Extended Data Fig. 2, panel f
Unprocessed western blots
Source Data Extended Data Fig. 2, panel f
Unprocessed western blots
Source Data Extended Data Fig. 2, panel f
Unprocessed western blots
Source Data Extended Data Fig. 2, panel f
Unprocessed western blots
Source Data Extended Data Fig. 2, panel k
Unprocessed western blots
Source Data Extended Data Fig. 2, panel k
Unprocessed western blots
Source Data Extended Data Fig. 2, panel k
Unprocessed western blots
Source Data Extended Data Fig. 2, panel k
Unprocessed western blots
Source Data Extended Data Fig. 2, panel l
Unprocessed western blots
Source Data Extended Data Fig. 2, panel l
Unprocessed western blots
Source Data Extended Data Fig. 2, panel l
Unprocessed western blots
Source Data Extended Data Fig. 2, panel l
Unprocessed western blots
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
DeMichele, A., Clark, A.S., Shea, E. et al. Targeting dormant tumor cells to prevent recurrent breast cancer: a randomized phase 2 trial. Nat Med (2025). https://doi.org/10.1038/s41591-025-03877-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-025-03877-3