Abstract
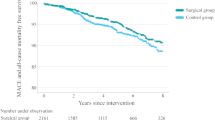
Both metabolic surgery and glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) improve cardiometabolic outcomes, but their long-term outcomes have not been directly compared. Here, we compared macrovascular and microvascular outcomes in 1,657 patients (65.7% female) with type 2 diabetes and obesity who underwent metabolic surgery with 2,275 similar patients (53.5% female) who received treatment with GLP-1 RAs. Using a doubly robust estimation method to balance baseline characteristics between groups, we examined the time to all-cause mortality, incident major adverse cardiovascular events (MACE), nephropathy and retinopathy over a median follow-up of 5.9 years. The 10-year cumulative incidence of all-cause mortality was 9.0% (95% confidence interval (CI) 6.8–10.8%) in the metabolic surgery group and 12.4% (95% CI 9.9–15.2%) in the GLP-1 RA group (adjusted hazard ratio (HR) 0.68 (95% CI 0.48–0.96), P = 0.028). Compared with the GLP-1 RA group, metabolic surgery was also associated with a lower risk of MACE (adjusted HR 0.65; 95% CI 0.51–0.82; P < 0.001), nephropathy (adjusted HR 0.53; 95% CI 0.43–0.67; P < 0.001) and retinopathy (adjusted HR 0.46; 95% CI 0.29–0.75; P = 0.002). These findings indicate that even with the availability of GLP-1 RAs, metabolic surgery remains superior to medical treatment. Future studies should compare the cardiometabolic outcomes of metabolic surgery with newer GLP-1 RAs that are more effective for weight reduction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The dataset generated during the current study is not publicly available to protect patient confidentiality. However, it is available to academic investigators upon request, pending the receipt of a signed data sharing agreement and review of the study protocol (approved by a local institutional review board or research ethics committee), statistical analysis plan and publication plan. All data sharing requests must be approved by the CCHS institutional review board and the Law Department before de-identified data can be shared. The corresponding authors will respond to requests within 2 months of receipt.
References
Courcoulas, A. P. et al. Long-term outcomes of medical management vs bariatric surgery in type 2 diabetes. JAMA 331, 654–664 (2024).
Mingrone, G. et al. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386, 964–973 (2015).
Ikramuddin, S. et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA 319, 266–278 (2018).
Aminian, A. et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 322, 1271–1282 (2019).
Fisher, D. P. et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA 320, 1570–1582 (2018).
O’Brien, R. et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann. Intern. Med. 169, 300–310 (2018).
Wolff Sagy, Y. et al. Effectiveness of bariatric metabolic surgery versus glucagon-like peptide-1 receptor agonists for prevention of congestive heart Failure. Nat. Med. 30, 2337–2342 (2024).
American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2020. Diabetes Care 43, S111–S134 (2020).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389, 2221–2232 (2023).
Stenberg, E. & Näslund, E. Major adverse cardiovascular events among patients with type-2 diabetes, a nationwide cohort study comparing primary metabolic and bariatric surgery to GLP-1 receptor agonist treatment. Int. J. Obes. 47, 251–256 (2023).
Thomas, L. E., Li, F. & Pencina, M. J. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 323, 2417–2418 (2020).
Funk, M. J. et al. Doubly robust estimation of causal effects. Am. J. Epidemiol. 173, 761–767 (2011).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Trujillo, J. M., Nuffer, W. & Smith, B. A. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 12, 204201882199732 (2021).
Douros, J. D., Tong, J. & D’Alessio, D. A. The effects of bariatric surgery on islet function, insulin secretion, and glucose control. Endocr. Rev. 40, 1394–1423 (2019).
Batterham, R. L. & Cummings, D. E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 39, 893–901 (2016).
Gasoyan, H., Pfoh, E. R., Schulte, R., Le, P. & Rothberg, M. B. Early‐ and later‐stage persistence with antiobesity medications: a retrospective cohort study. Obesity 32, 486–493 (2024).
Liss, D. T. et al. Treatment modification after initiating second-line medication for type 2 diabetes. Am. J. Manag. Care 29, 661–668 (2023).
Wilding, J. P. H. et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes. Metab. 24, 1553–1564 (2022).
Bashir, B. et al. Microvascular complications of obesity and diabetes—role of bariatric surgery. Obes. Rev. 24, e13602 (2023).
Vivante, A. et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch. Intern. Med. 172, 1644–1650 (2012).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Davies, M. et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 397, 971–984 (2021).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Gasoyan, H. et al. One-year weight reduction with semaglutide or liraglutide in clinical practice. JAMA Netw. Open 7, e2433326 (2024).
Hernán, M. A., Wang, W. & Leaf, D. E. Target trial emulation: a framework for causal inference from observational data. JAMA 328, 2446–2447 (2022).
Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 183, 758–764 (2016).
Madenci, A. L. et al. Estimating the effect of bariatric surgery on cardiovascular events using observational data? Epidemiology 35, 721–729 (2024).
VanderWeele, T. J. & Ding, P. Sensitivity analysis in observational research: introducing the E-value. Ann. Intern. Med. 167, 268–274 (2017).
Inker, L. A. et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 385, 1737–1749 (2021).
Blecker, S. et al. Validation of EHR medication fill data obtained through electronic linkage with pharmacies. J. Manag. Care Spec. Pharm. 27, 1482–1487 (2021).
Milinovich, A. & Kattan, M. W. Extracting and utilizing electronic health data from Epic for research. Ann. Transl. Med. 6, 42 (2018).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349 (2008).
Van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Rubin, D. B. Inference and missing data. Biometrika 63, 581–592 (1976).
Austin, P. C. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J. Clin. Epidemiol. 63, 46–55 (2010).
Raebel, M. A., Schmittdiel, J., Karter, A. J., Konieczny, J. L. & Steiner, J. F. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med. Care 51, S11–S21 (2013).
Acknowledgements
We thank M. E. Satava from the Cleveland Clinic, for her help in collecting some of the data. She did not receive additional compensation, outside her usual salary, for her contribution.
Author information
Authors and Affiliations
Contributions
H.G., S.E.N., M.B.R. and A.A. contributed to study conception and design. H.G., M.H.A., N.J.C., A.A.J., N.D., H.J. and A.A. were involved in data acquisition. A.Z. performed the statistical analysis. All authors contributed to the interpretation of the data. H.G. and A.A. prepared the first draft of the manuscript. All authors critically revised the manuscript for intellectual content and clarity, and approved the final version for submission. A.A. provided administrative support and supervised the work.
Corresponding authors
Ethics declarations
Competing interests
W.S.B. has received honoraria and paid consultancy from Novo Nordisk, Abbott Nutrition, Medscape, Alfie Health and the Med Learning Group. R.P.S. reported receiving personal fees from Alcon, Apellis, Bausch + Lomb, EyePoint, Genentech, Iveric Bio, Regeneron, Regenxbio and ZEISS, and research grants from Janssen. W.H.W.T. received consulting fees from Sequana Medical, Cardiol Therapeutics, Genomics plc, Zehna Therapeutics, WhiteSwell, Boston Scientific, CardiaTec Biosciences, Intellia Therapeutics, Bristol Myers Squibb, Alleviant Medical, Alexion Pharmaceuticals, Salubris Biotherapeutics and BioCardia, and received honoraria from Springer, Belvoir Media Group and the American Board of Internal Medicine. B.B. received an honorarium from Novo Nordisk. R.J.R. reported receiving personal fee from Medtronic, Diagnostic Green, mediCAD and Dendrite Imaging, and serving as the CEO of Dendrite Imaging. S.E.N. received grants to perform clinical trials from AbbVie, AstraZeneca, Amgen, Bristol Myers Squibb, Eli Lilly, Esperion Therapeutics, Medtronic, MyoKardia, New Amsterdam Pharmaceuticals, Novartis and Silence Therapeutics. M.B.R. has a consulting relationship with the Blue Cross Blue Shield Association. A.A. received research grants from Medtronic and Ethicon, and serves as a consultant for Medtronic, Ethicon and Eli Lilly. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Anne Ehlers, Abdelrahmin Nimeri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
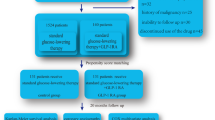
Extended Data Fig. 1 Identification of eligible patients for inclusion.
Details of International Classification of Diseases (ICD) and Current Procedural Terminology (CPT) codes used in cohort construction are available in Supplementary Table 3. a Five dates from the collection of index dates of surgical patients were randomly assigned to each nonsurgical control, which served as potential index dates for nonsurgical patients. b Some patients met multiple exclusion criteria. c In case of more than one index date that a patient would be eligible for inclusion, we selected the earliest eligible index date for each nonsurgical patient. Abbreviations: BMI, body mass index; ED, emergency department; eGFR, estimated glomerular filtration rate; GLP-1RA - glucagon-like peptide-1 receptor agonist; T2DM, type 2 diabetes mellitus.
Extended Data Fig. 2 Proportions of patients with orders and dispenses for other medications.
Plots display proportions of patients (with 95% point-wise confidence intervals (shaded areas)) in the metabolic surgery and GLP-1RA groups over time who were prescribed or dispensed with the indicated medications. For a given time point (e.g., 1 year after the index date), prescription orders (dashed lines) were defined as the percentage of patients (of those who were followed up to at least that date) who had at least one prescription order for that medication, with start and end dates that encompassed that time point (using the last follow-up date if the end date was missing). For a given time point (e.g., 1 year after the index date), medication dispenses (solid lines) were defined as the percentage of patients (of those who were followed up to at least that date) who had at least one dispense record for that medication in the preceding 6 months. Medication dispensation data was based on Surescripts data. SGLT2, Sodium-Glucose Cotransporter 2.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gasoyan, H., Alavi, M.H., Zajichek, A. et al. Macrovascular and microvascular outcomes of metabolic surgery versus GLP-1 receptor agonists in patients with diabetes and obesity. Nat Med 31, 3341–3349 (2025). https://doi.org/10.1038/s41591-025-03893-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03893-3
This article is cited by
-
Peptidhormonanaloga basierte medikamentöse Adipositastherapie ist effektiv
Die Chirurgie (2026)