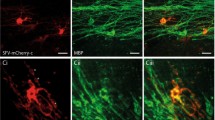
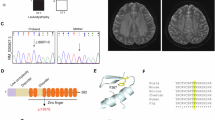
Abstract
This open-label phase 1/2 clinical study uses a novel recombinant vector, rAAV-Olig001, with selective tropism for oligodendrocytes, to deliver gene therapy for Canavan disease (CD), a rare leukodystrophy characterized by defective aspartoacylase and elevated N-acetyl-aspartic acid (NAA) concentrations. A total of 8 participants received intracranial doses of 3.7 × 1013 vector genomes (vg) of rAAV-Olig001-ASPA (MYR-101), with an interim analysis at 12 months. The primary objective was to assess the safety of intracranial dosing of MYR-101 in children with typical CD. Efficacy measures included Mullen Scales of Early Learning (MSEL), Gross Motor Function Measure (GMFM) and analysis of NAA, myelination, white matter and extracellular water content in the brain. The participants were White; 5 (62.5%) were male. Of the participants, 7 (87.5%) experienced ≥1 serious adverse event, none of which were considered MYR-101 related. All participants experienced ≥1 adverse event. All adverse events and serious adverse events resolved fully. Treatment reduced NAA concentrations in cerebrospinal fluid (P = 0.0008), increased myelination (P = 0.0137) and improved MSEL developmental outcomes (P = 0.0171). Thus, interim results suggest that gene therapy with MYR-101 is well tolerated and shows early effects in CD. While these findings are preliminary, reductions in NAA concentrations indicate ASPA expression and increases in myelination and imply successful targeting of oligodendrocytes. These results may support the development of similar gene therapy strategies for other demyelinating and metabolic brain disorders. ClinicalTrials.gov registration: NCT04833907.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
At the outset of the trial, we omitted a data-sharing provision from the consent documents signed by participants. As a result, in accordance with our Ethics Committee policies, we are not authorized to release the raw data to the public. Furthermore, the study is still in progress. De-identified patient characteristics, safety and preliminary efficacy data from raw datasets generated in this study are included in the paper. Requests for more information about the raw data are subject to a confidentiality agreement with Myrtelle and must comply with applicable legal and regulatory requirements. Qualified researchers may request access to the trial information by contacting corresponding author O.F. The requests will be addressed within 120 days, and data transfer agreement may be required.
References
Canavan, M. M. Schilder’s encephalitis periaxialis diffusa: report of a case of a child aged sixteen and one-half months. Arch. Neurol. Psychiatry 25, 299–308 (1931).
Leone, P. et al. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann. Neurol. 48, 27–38 (2000).
Matalon, R., Kaul, R. & Michals, K. Canavan disease: biochemical and molecular studies. J. Inherit. Metab. Dis. 16, 744–752 (1993).
Baslow, M. H. Canavan’s spongiform leukodystrophy: a clinical anatomy of a genetic metabolic CNS disease. J. Mol. Neurosci. 15, 61–69 (2000).
Francis, J. S. et al. N-Acetylaspartate supports the energetic demands of developmental myelination via oligodendroglial aspartoacylase. Neurobiol. Dis. 96, 323–334 (2016).
Hoshino, H. & Kubota, M. Canavan disease: clinical features and recent advances in research. Pediatr. Int. 56, 477–483 (2014).
Lotun, A., Gessler, D. J. & Gao, G. Canavan disease as a model for gene therapy-mediated myelin repair. Front. Cell. Neurosci. 15, 661928 (2021).
Janson, C. G. et al. Mild-onset presentation of Canavan’s disease associated with novel G212A point mutation in aspartoacylase gene. Ann. Neurol. 59, 428–431 (2006).
Velinov, M., Zellers, N., Styles, J. & Wisniewski, K. Homozygosity for mutation G212A of the gene for aspartoacylase is associated with atypical form of Canavan’s disease. Clin. Genet. 73, 288–289 (2008).
Adachi, M., Schneck, L., Cara, J. & Volk, B. W. Spongy degeneration of the central nervous system (van Bogaert and Bertrand type; Canavan’s disease). A review. Hum. Pathol. 4, 331–347 (1973).
Matalon, R. et al. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am. J. Med. Genet. 29, 463–471 (1988).
Traeger, E. C. & Rapin, I. The clinical course of Canavan disease. Pediatr. Neurol. 18, 207–212 (1998).
Bokhari, M. R., Samanta, D. & Bokhari, S. R. A. Canavan Disease (StatPearls Publishing, 2024).
Zelnik, N. et al. Protracted clinical course for patients with Canavan disease. Dev. Med Child Neurol. 35, 355–358 (1993).
Zeng, B. J. et al. Identification and characterization of novel mutations of the aspartoacylase gene in non-Jewish patients with Canavan disease. J. Inherit. Metab. Dis. 25, 557–570 (2002).
Janson, C. et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 13, 1391–1412 (2002).
Leone, P. et al. Long-term follow-up after gene therapy for Canavan disease. Sci. Transl. Med. 4, 165ra3 (2012).
Francis, J. S. et al. Preclinical biodistribution, tropism, and efficacy of oligotropic AAV/Olig001 in a mouse model of congenital white matter disease. Mol. Ther. Methods Clin. Dev. 20, 520–534 (2021).
Traka, M. et al. Nur7 is a nonsense mutation in the mouse aspartoacylase gene that causes spongy degeneration of the CNS. J. Neurosci. 28, 11537–11549 (2008).
Hull, V. et al. Antisense oligonucleotide reverses leukodystrophy in Canavan disease mice. Ann. Neurol. 87, 480–485 (2020).
Maier, H., Wang-Eckhardt, L., Hartmann, D., Gieselmann, V. & Eckhardt, M. N-Acetylaspartate synthase deficiency corrects the myelin phenotype in a Canavan disease mouse model but does not affect survival time. J. Neurosci. 35, 14501–14516 (2015).
Pleasure, D. et al. Pathophysiology and treatment of Canavan disease. Neurochem. Res. 45, 561–565 (2020).
McAllister, A. et al. Quantitative synthetic MRI in children: normative intracranial tissue segmentation values during development. Am. J. Neuroradiol. 38, 2364–2372 (2017).
Janson, C. G. et al. Natural history of Canavan disease revealed by proton magnetic resonance spectroscopy (1H-MRS) and diffusion-weighted MRI. Neuropediatrics 37, 209–221 (2006).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Eng. J. Med. 377, 1713–1722 (2017).
Whitley, C. B. et al. Final results of the phase 1/2, open-label clinical study of intravenous recombinant human N-acetyl-α-d-glucosaminidase (SBC-103) in children with mucopolysaccharidosis IIIB. Mol. Genet. Metab. 126, 131–138 (2019).
Jakobs, C. et al. Stable isotope dilution analysis of N-acetylaspartic acid in CSF, blood, urine and amniotic fluid: accurate postnatal diagnosis and the potential for prenatal diagnosis of Canavan disease. J. Inherit. Metab. Dis. 14, 653–660 (1991).
Kolodziejczyk, K., Hamilton, N. B., Wade, A., Káradóttir, R. & Attwell, D. The effect of N-acetyl-aspartyl-glutamate and N-acetyl-aspartate on white matter oligodendrocytes. Brain 132, 1496–1508 (2009).
Corti, M. et al. Adeno-associated virus-mediated gene therapy in a patient with Canavan disease using dual routes of administration and immune modulation. Mol. Ther. Methods Clin. Dev. 30, 303–314 (2023).
Bley, A. et al. The natural history of Canavan disease: 23 new cases and comparison with patients from literature. Orphanet J. Rare Dis. 16, 227 (2021).
Janson, C. G., Romanova, L. G., Rudser, K. D. & Haines, S. J. Improvement in clinical outcomes following optimal targeting of brain ventricular catheters with intraoperative imaging. J. Neurosurg. 120, 684–696 (2014).
Acknowledgements
We thank the patients and families who generously contributed their time, courage and commitment to this research that made this study possible. We also wish to thank all of the Canavan Disease Patient Advocacy Groups worldwide for their commitment to research and patient care in this community, and the entire team at Dayton Children's Hospital for their support and collaboration. We sincerely thank S. Hesterlee, now interim president and CEO of the Muscular Dystrophy Association, for her exceptional guidance and support during the early development of this program in her former role as program director of the Canavan Disease Program. We also thank L. E. Kratz and the Kennedy Krieger Institute Biochemical Genetics Lab for analyzing the CSF NAA samples. We thank A. Pace for statistical support on this project, particularly with MSEL statistical analysis. We also wish to acknowledge the important scientific contributions of J. R. Samulski and S. Gray, whose expertise has been instrumental during the first phase of this program. We gratefully acknowledge the members of the Independent Data Monitoring Committee for their expert guidance, oversight and commitment to ensuring the integrity of the trial. Their independent review and thoughtful recommendations have been invaluable in guiding the conduct of this study. We thank the FDA for their supportive guidance and for recognizing the urgent, unmet needs of patients and families affected by this devastating disease and their commitment to accelerating the progress of this program. This study is funded by Myrtelle, Inc. Paper preparation support, provided by J. G. Jacobson, InSeption Group, was also funded by Myrtelle, Inc.
Author information
Authors and Affiliations
Contributions
P.L. contributed to study design, data interpretation, revision of the paper and approval of the paper for submission. R.M.L. contributed to data interpretation, revision of the paper and approval of the paper for submission. J.F. contributed to data interpretation, revision of the paper and approval of the paper for submission. O.F. contributed to data interpretation, writing and revision of the paper and approval of the paper for submission. C.G.J. contributed to data analysis and interpretation, writing and revision of the paper, approval of the paper for submission. D.S. contributed to statistical analyses, revision of the paper and approval of the paper for submission. K.M.C. contributed to data interpretation, revision of the paper and approval of the paper for submission.
Corresponding authors
Ethics declarations
Competing interests
P.L. is a shareholder of and paid consultant for Myrtelle, Inc. R.M.L. and J.F. report no conflicts of interest. C.G.J., D.S. and K.M.C. are paid consultants for Myrtelle, Inc. O.F. is co-chief medical officer and a shareholder of Myrtelle, Inc.
Peer review
Peer review information
Nature Medicine thanks Evan Snyder and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Change in N-acetyl-aspartic acid (NAA) concentrations in the brain, measured by magnetic resonance spectroscopy after MYR-101 treatment.
Panel a. Mean change in NAA across the occipital region for individual participants (average of right and left occipital voxels), measured by MRS. Population fit (i.e., mean line for the entire population) is shown in red. For reference, normal age-matched NAA concentrations in the occipital lobe range from 4-7 mM. Panel b. individual NAA values for each participant, including participant for whom pre-treatment values were available; in those participants, NAA slope was positive leading up to gene therapy and negative afterwards, as previously described2.
Extended Data Fig. 2 Participant-level changes in myelin as a function of time from treatment.
Myelin volume is shown in mLs. Participants are identified by their age at time of treatment.
Extended Data Fig. 3 Change in Gross Motor Function Measure (GMFM-88) raw scores in treated participants by chronological age.
Panel a: Change in individual participants on the Lying and Rolling domain of the Gross Motor Function Measure. Population fit (mean line for the entire population) is shown in red. Panel b: Change in individual participants on the Sitting domain of the Gross Motor Function Measure. Population fit is shown in red.
Supplementary information
Supplementary Information
Supplementary methods.
Supplementary Video 1
A child with typical Canavan disease before treatment.
Supplementary Video 2
Video 1 of the same child after treatment.
Supplementary Video 3
Video 2 of the same child after treatment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leone, P., Lober, R.M., Francis, J. et al. Oligodendrocyte-targeted adeno-associated virus gene therapy for Canavan disease in children: a phase 1/2 trial. Nat Med 31, 3772–3779 (2025). https://doi.org/10.1038/s41591-025-03919-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03919-w