Abstract
In DESTINY-Breast04 (NCT03734029), trastuzumab deruxtecan (T-DXd) significantly improved overall survival (OS) and progression-free survival compared with treatment of physician’s choice of chemotherapy (TPC) for patients with human epidermal growth factor receptor 2-low (HER2-low) (immunohistochemistry (IHC) 1+ or IHC 2+/in situ hybridization-negative) metastatic breast cancer. After an extended median follow-up of 32.0 months, median OS in the overall cohort was 22.9 months for T-DXd and 16.8 months for TPC (hazard ratio 0.69; 95% confidence interval 0.55–0.86). For the hormone receptor-positive cohort, median OS was 23.9 and 17.6 months for T-DXd and TPC, respectively (hazard ratio 0.69; 95% confidence interval 0.55–0.87). Median OS also favored T-DXd in exploratory analyses of hormone receptor-negative, estrogen receptor IHC 1%–10% and estrogen receptor IHC >10% cohorts. The overall safety profile of T-DXd was acceptable and generally manageable. Results confirm T-DXd as standard of care after prior chemotherapy in patients with HER2-low metastatic breast cancer. ClinicalTrials.gov identifier: NCT03734029.
Similar content being viewed by others
Main
Human epidermal growth factor receptor 2 (HER2; encoded by ERBB2) expression and hormone receptor (HR) status are tumor characteristics that influence the outcomes and guide treatment strategies for invasive breast cancer1. As a result, these markers are recognized by international guidelines, and determining their status is an essential step for all patients with breast cancer1,2,3,4. HR status is defined by the presence of tumor nuclei staining by IHC for estrogen receptor (ER) and/or progesterone receptor (PR)2. The expression and gene amplification status of HER2 in breast cancer is defined using IHC and in situ hybridization (ISH)3. The American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) clinical practice guidelines on HER2 testing in breast cancer classify tumors as HER2-positive (HER2+; defined as an IHC score of 3+ or IHC 2+/ISH+) or HER2-negative (HER2−; IHC 0, IHC 1+ or IHC 2+/ISH−)3,5,6. The HER2-low category, defined as an IHC score of 1+ or IHC 2+/ISH−, was introduced after results from a phase 1b study (NCT02564900) of T-DXd and primary results of the DESTINY-Breast04 trial were released7,8,9. The updated 2023 ASCO/CAP guidelines recognize the development of new antibody–drug conjugates (ADCs), such as T-DXd, for breast cancers that lack HER2 protein overexpression or gene amplification. The updated guidelines also state that, although HER2-low is currently not considered a new biological category, it is now best practice to distinguish IHC 0 from IHC 1+ on pathology reports in view of its diagnostic and therapeutic impact5. Guidelines worldwide have also adopted these changes10,11. Based on retrospective studies of primary or metastatic breast cancer, approximately 65% of tumors originally classified as HER2− may now meet HER2-low criteria, providing opportunities for improved treatment selection for patients with breast cancer12,13.
Patients with HR+ breast cancer (defined by tumor samples having 1%–100% of tumor nuclei staining positive for ER and/or PR) generally have a better prognosis than those diagnosed with HR− tumors (defined by <1% staining)2,14. A new reporting category per ASCO/CAP guidelines has been suggested for cancers with 1%–10% ER tumor nuclei staining because of studies showing that patients with ER-low-positive metastatic breast cancer are unlikely to benefit from endocrine therapy and have a similar response to cancer treatment as HR− breast cancer2,15,16,17,18,19.
DESTINY-Breast04 is an open-label, randomized, phase 3 clinical trial designed to evaluate the efficacy and safety of T-DXd compared with TPC (capecitabine, eribulin, gemcitabine, paclitaxel or nab-paclitaxel) for patients with HR+ or HR−, HER2-low, unresectable or metastatic breast cancer previously treated with one or two lines of chemotherapy in the metastatic setting8. T-DXd is an ADC composed of a HER2-directed, humanized immunoglobulin G1 monoclonal antibody linked to a potent topoisomerase I inhibitor that targets tumor cells with different levels of HER2 expression in addition to neighboring cells through a bystander antitumor effect20,21. The DESTINY-Breast04 primary analysis (data cutoff 11 January 2022) showed that T-DXd significantly improved progression-free survival (PFS) by blinded independent central review (BICR), the primary endpoint (in the HR+ cohort), and OS compared with TPC8. T-DXd was also associated with an acceptable and generally manageable safety profile.
Based on the DESTINY-Breast04 results, T-DXd became the first HER2-directed therapy to show clinical efficacy in HER2-low breast cancer, thereby identifying a new clinically targetable patient population. T-DXd has since been approved by regulatory bodies including the US Food and Drug Administration for patients with HER2-low unresectable or metastatic breast cancer, and DESTINY-Breast04 demonstrated the importance of considering HER2 IHC and/or ISH results in the HER2-low category in the selection of further lines of therapy among patients with disease progression on standard treatment8,22. Here we report a preplanned updated OS analysis, long-term safety and exploratory analyses of HR−, ER-low-positive and ER IHC >10% subgroups from DESTINY-Breast04 after longer follow-up (data cutoff 1 March 2023).
Results
Patient characteristics
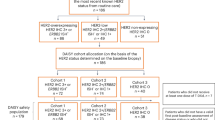
Following screening between 27 December 2018 and 31 December 2021, 577 patients with centrally confirmed HER2-low metastatic breast cancer were randomly assigned to receive either T-DXd (n = 373) or TPC (n = 184)8 (Fig. 1a). At the updated data cutoff date with a median follow-up of 32.0 months (95% confidence interval (CI) 31.0–32.8), 17 patients (4.6%) remained on T-DXd treatment, whereas none of the patients assigned to the TPC arm continued study treatment (Fig. 1a). The main reason for treatment discontinuation in both treatment arms was disease progression; 256 patients (69.0%) and 132 patients (76.7%) experienced disease progression in the T-DXd and TPC arms, respectively. Demographics and baseline characteristics for the overall and HR cohorts are presented in Table 1.
Patient disposition (a) and OS in the overall cohort (b). Percentages depicted in the patient disposition figure are based on the number of patients in each arm. ‘Other’ in the patient disposition figure includes clinical progression, physician decision, lost to follow-up and other unknown reasons. For the OS and PFS by investigator endpoints for the HR+ and all patient cohorts, the HR status is based on data collected using the interactive web-response and voice-response system at the time of randomization, which includes patients who were misstratified. The dashed lines in the Kaplan-Meier curve depict the landmark OS rates at 24 and 36 months.
Efficacy
DESTINY-Breast04 achieved statistical significance for T-DXd versus TPC on its primary endpoint (PFS by BICR) in the HR+ cohort, as well as key secondary endpoints (PFS by BICR in the overall cohort (HR+ and HR−) and OS in the overall and HR+ cohorts) at the time of the primary analysis8. Because the statistical boundary for the primary and secondary endpoints was crossed during the primary analysis, BICR analysis was concluded. For this updated data cutoff (1 March 2023), analyses included OS and PFS by investigator in the overall and HR+ cohorts. We also conducted exploratory analyses of OS and PFS by investigator in the HR− cohort and ER expression subgroups (IHC 1%–10%; IHC >10%).
OS results were consistent with those from the primary analysis, with a median OS in the overall cohort of 22.9 months (95% CI 21.2–24.5) for T-DXd and 16.8 months (95% CI 14.1–19.5) for TPC (hazard ratio 0.69; 95% CI 0.55–0.86) (Fig. 1b). For the HR+ cohort, the median OS was 23.9 months (95% CI 21.7–25.2) in the T-DXd arm and 17.6 months (95% CI 15.1–20.2) in the TPC arm (hazard ratio 0.69; 95% CI 0.55–0.87) (Fig. 2a). In the HR− cohort, median OS was 17.1 months (95% CI 13.6–23.0) in the T-DXd arm and 8.3 months (95% CI 5.6–20.4) in the TPC arm (hazard ratio 0.58; 95% CI 0.31–1.08) (Fig. 2b). The OS rate at 24 months was more than two times greater in the T-DXd arm compared with the TPC arm (32.6% versus 11.8%, respectively).
a,b, HR+ cohort (a) and HR− cohort (b). For the OS and PFS by investigator endpoints for the HR+ and all patient cohorts, the HR status is based on data collected using the interactive web-response and voice-response system at the time of randomization, which includes patients who were misstratified. The dashed lines depict the landmark OS rates at 24 and 36 months.
T-DXd demonstrated OS benefit compared with TPC across all demographic subgroups in the overall and HR+ cohorts (Extended Data Fig. 1a,b). In the overall cohort, the efficacy of T-DXd was consistent by IHC status. Moreover, patients in the overall cohort who had prior treatment with cyclin-dependent kinase (CDK) 4 and CDK6 inhibitors presented a median OS of 22.3 months (95% CI 19.7–24.2) in the T-DXd arm and 16.7 months (95% CI 14.0–19.4) in the TPC arm.
The median PFS by investigator assessment was 8.8 months (95% CI 8.3–9.8) for T-DXd versus 4.2 months (95% CI 3.0–4.5) for TPC (hazard ratio 0.36; 95% CI 0.29–0.45) in the overall cohort (Extended Data Fig. 2). The PFS rate at 24 months was 14.5% (95% CI 10.8–18.7) for T-DXd. For the HR+ cohort, the median PFS by investigator was 9.6 months (95% CI 8.4–10.0) for T-DXd versus 4.2 months (95% CI 3.4–4.9) for TPC (hazard ratio 0.37; 95% CI 0.30–0.46) (Extended Data Fig. 3a). Median PFS by investigator in the HR− cohort was 6.3 months (95% CI, 4.2–8.5) for T-DXd versus 2.9 months (95% CI, 1.4–4.2) for TPC (hazard ratio 0.29; 95% CI 0.15–0.57) (Extended Data Fig. 3b).
Median PFS from time of randomization to progression on next line of therapy or death (PFS2) by investigator assessment for the overall and HR+ cohorts are shown in Table 2. In the overall cohort, PFS2 was 15.4 months (95% CI 13.6–16.5) in the T-DXd arm and 9.7 months (95% CI 8.3–10.8) in the TPC arm. In the HR+ cohort, PFS2 was 15.5 months (95% CI 13.8–17.2) and 10.5 months (95% CI 8.3–11.4) in the T-DXd and TPC arms, respectively. In the HR− cohort exploratory analysis, median PFS2 was 12.9 months (95% CI 9.6–18.3) and 6.7 months (95% CI 5.0–12.7) in the T-DXd and TPC arms, respectively.
In the overall cohort, the most common first post-trial anticancer therapies received by patients in the T-DXd and TPC arms are shown in Table 2. In the HR+ cohort, the most common first post-trial therapy received by patients in the T-DXd and TPC arms included chemotherapy (156 patients (47.1%) versus 74 patients (45.4%)) and endocrine therapy (50 patients (15.1%) versus 20 patients (12.3%)), respectively; 8 patients (2.4%) in the T-DXd arm and 1 patient (0.6%) in the TPC arm received ADCs as first post-trial therapy.
In the overall cohort, 18 patients (4.8%) versus 15 patients (8.2%) received ADCs as any post-trial anticancer therapy in the T-DXd and TPC arms, respectively; for the HR+ cohort, 16 patients (4.8%) in the T-DXd arm and 15 patients (9.2%) in the TPC arm received ADCs as any post-trial anticancer therapy (Extended Data Table 1).
In the exploratory efficacy analyses of both ER expression subgroups (ER >10% and ER-low-positive (IHC 1%–10%)), the median OS and PFS by investigator assessment favored the T-DXd arm compared with the TPC arm (Extended Data Table 2). Median OS was 24.0 months (95% CI 21.7–25.7) for T-DXd versus 18.9 months (95% CI 16.0–22.3) for TPC in the ER >10% subgroup (hazard ratio 0.72; 95% CI 0.56–0.93) and 22.3 months (95% CI 16.6–27.1) for T-DXd versus 10.2 months (95% CI 7.8–14.5) for TPC in the ER-low-positive subgroup (hazard ratio 0.40; 95% CI 0.20–0.79). Median PFS was 9.6 months (95% CI 8.4–10.9) for T-DXd versus 4.3 months (95% CI 4.0–5.3) for TPC in the ER >10% subgroup (hazard ratio 0.37; 95% CI 0.29–0.47) and 8.3 months (95% CI 5.6–10.2) for T-DXd versus 2.3 months (95% CI 1.3–4.1) for TPC in the ER-low-positive subgroup (hazard ratio 0.27; 95% CI 0.14–0.52).
An exploratory efficacy analysis was also conducted on the overall cohort based on best confirmed response to T-DXd treatment per investigator assessment. Patients who achieved a complete response in the T-DXd arm (n = 8) had a median PFS by investigator assessment of 25.3 months (95% CI 6.9 to not evaluable (NE)) and OS was NE (95% CI 20.3 to NE) (Fig. 3a,b). Median PFS by investigator assessment and OS in patients with a partial response in the T-DXd arm (n = 188) were 11.1 months (95% CI, 9.7–12.6) and 24.7 months (95% CI, 23.0–30.4), respectively.
Finally, an exploratory subgroup analysis was conducted in the HR+ cohort in which OS was compared between T-DXd and the individual chemotherapy treatments included in TPC. The median OS was numerically longer with T-DXd compared with all the TPC treatment options (investigator assignment before randomization), with hazard ratios ranging from 0.23 to 0.80 (Extended Data Table 3).
Safety
The safety profile of T-DXd was consistent with the results from the primary analysis (Table 3). Median treatment duration at the updated data cutoff was 8.2 months (range 0.2–39.1 months) in the T-DXd arm and 3.5 months (range 0.3–19.7 months) in the TPC arm. The rate of any-grade treatment-emergent adverse events (TEAEs) was similar in both arms, with 99.5% in the T-DXd arm and 98.3% in the TPC arm. Grade ≥3 TEAEs occurred in 202 patients (54.4%) in the T-DXd arm and in 116 patients (67.4%) in the TPC arm. In the T-DXd and TPC arms, 62 (16.7%) and 14 patients (8.1%), respectively, discontinued treatment because of TEAEs.
Exposure-adjusted incidence rates (EAIRs) were measured to account for the differences in treatment duration exposure between the treatment arms. EAIRs for any-grade TEAEs were 1.16 and 2.64 per patient-year for the T-DXd and TPC arms, respectively, while for grade ≥3 TEAEs they were 0.64 with T-DXd and 1.81 with TPC. EAIRs for TEAEs associated with treatment discontinuation were 0.20 and 0.22 in the T-DXd and TPC arms, respectively (Extended Data Table 4).
The most common TEAEs associated with drug discontinuation were investigator-assessed interstitial lung disease (ILD) and/or pneumonitis at 10.2% in the T-DXd arm and peripheral sensory neuropathy at 2.3% in the TPC arm. The most common any-grade drug-related TEAEs in the T-DXd arm were nausea (76.0%), fatigue (55.0%), vomiting (40.7%) and alopecia (39.9%), compared with neutropenia (52.3%), fatigue (48.8%), leukopenia (32.6%) and increased transaminases (31.4%) in the TPC arm (Extended Data Table 5). The most common grade ≥3 drug-related TEAEs in the T-DXd arm were neutropenia (14.6%), anemia (10.8%), fatigue (9.7%) and leukopenia (7.0%), compared with neutropenia (42.4%), leukopenia (19.2%) and increased transaminases (13.4%) in the TPC arm.
Adverse events of special interest included ILD and/or pneumonitis and left ventricular dysfunction (Extended Data Table 6). After an additional ~14 months of follow-up since the data cutoff of the primary analysis (11 January 2022), no new cases of ILD and/or pneumonitis were reported. Adjudicated drug-related ILD and/or pneumonitis was observed in 45 patients (12.1%) in the T-DXd arm; median time to onset of ILD was 129 days (range 26–710). Most events were lower grade, with 13 patients (3.5%) having a grade 1 event, 24 patients (6.5%) having a grade 2 event, 4 patients (1.1%) having a grade 3 event, 0 patients with a grade 4 event and 4 patients (1.1%) having a grade 5 event. One of the adjudicated drug-related grade 5 events in the updated analysis was a grade 3 event at the time of the primary analysis. In the TPC arm, adjudicated drug-related ILD and/or pneumonitis was reported in one patient (0.6%; grade 1) who received eribulin; time to onset of ILD was 60 days. For left ventricular dysfunction, 19 patients (5.1%) in the T-DXd arm experienced an any-grade event. There were no left ventricular dysfunction events in the TPC arm. Of the 19 patients in the T-DXd arm, 18 (4.9%) experienced decreased ejection fraction: 2 patients (0.5%) experienced a grade 1 event, 15 (4.0%) experienced a grade 2 event and 1 (0.3%) experienced a grade 3 event. Cardiac failure was experienced by 2 patients (0.5%): 1 patient (0.3%) experienced grade 2 and another (0.3%) experienced grade 3 cardiac failure. The patient who experienced the grade 3 cardiac failure also experienced a grade 2 decreased ejection fraction.
Discussion
Results from the updated analysis of DESTINY-Breast04 confirm the sustained clinically meaningful benefit of T-DXd versus TPC as previously demonstrated in HR+ and HR−, HER2-low metastatic breast cancer. OS was improved with T-DXd compared with TPC and confirmed the results from the primary analysis8. Patients treated with T-DXd had a 31% reduction in the risk of death in both the overall and HR+ cohorts and a 42% reduction in risk of death in the exploratory HR− cohort.
T-DXd also showed better efficacy compared with TPC in terms of investigator-assessed PFS and PFS2, which were consistently longer in the T-DXd arm versus the TPC arm in the overall, HR+ and HR− cohorts. The increased median PFS2 seen with T-DXd suggests that T-DXd may not cause treatment resistance to subsequent therapies compared with TPC. In this updated analysis, PFS was assessed by investigators because analyses of PFS by BICR were concluded after the primary analysis8. Investigator-assessed PFS may introduce bias; however, there is a similarity between the PFS by BICR reported in the primary analysis8 and the PFS by investigator reported in this updated analysis, supporting sustained T-DXd activity at a longer follow-up.
The DESTINY-Breast04 trial used the ASCO/CAP definition for ER+ (tumor samples having 1%–100% positive staining); however, a new reporting category for cancer with 1%–10% cell staining, termed ER-low-positive, has been recommended by the ASCO/CAP guidelines because of increasing concern over effective treatment strategies for patients with ER-low-positive tumor expression2,15,17,23. We therefore carried out an exploratory subgroup analysis by ER expression (ER-low-positive and ER >10%) in patients with HER2-low metastatic breast cancer and demonstrated that T-DXd achieved better efficacy outcomes than TPC in both populations. Studies have shown that patients with ER-low-positive and HR− breast cancer have a similar response to cancer treatment, which is considerably worse than for those with HR+ breast cancer17,18,19. Despite the small sample size and differences in disease characteristics between treatment arms of the HR− subgroup, the HR− subgroup was included as an exploratory analysis because of the high unmet clinical need in this population.
When compared with TPC, results from the exploratory efficacy analyses show T-DXd to be as effective in the ER-low-positive and HR− subgroups as in the HR+ subgroup. In addition, median OS and PFS values were similar with T-DXd in the different ER expressing cohorts (median OS: 24.0 months in ER >10% and 22.3 months in ER-low-positive; median PFS: 9.6 months in ER >10% and 8.3 months in ER-low-positive) compared with TPC (median OS: 18.9 months in ER >10% and 10.2 months in ER-low-positive; median PFS: 4.3 months in ER >10% and 2.3 months in ER-low-positive). This suggests the efficacy of T-DXd is independent of the ER level in the tumor.
The exploratory efficacy analysis by best confirmed response to T-DXd showed patients experiencing complete response or partial response had more pronounced survival outcomes than nonresponders, especially for PFS. These findings were consistent with the results of the pooled analysis conducted in HER2-positive metastatic breast cancer from the DESTINY-Breast01, DESTINY-Breast02 and DESTINY-Breast03 trials, which also showed patients with complete response or partial response had better efficacy outcomes in terms of OS and PFS compared with nonresponders24.
With longer treatment duration, the overall safety profile of T-DXd was maintained and was consistent with the primary analysis8. Notably, EAIRs indicated no cumulative toxicities with longer follow-up. The most common TEAEs in both treatment arms were hematologic and gastrointestinal in nature. In the T-DXd arm, nausea and vomiting were two of the most common any-grade drug-related TEAEs. As per the NCCN Clinical Practice Guidelines in Oncology for antiemesis, fam-trastuzumab deruxtecan-nxki (T-DXd) is known to be of high emetic risk; the prevention and aggressive management of nausea and vomiting using prophylactic antiemetic regimens is important for the patient’s quality of life during fam-trastuzumab deruxtecan-nxki (T-DXd) treatment and is therefore recommended by clinical guidelines25,26. For adverse events of special interest, rates of left ventricular dysfunction were consistent with those previously observed8. ILD and/or pneumonitis rates remained unchanged with longer follow-up suggesting late-onset ILD to be uncommon with T-DXd. Proactive patient monitoring and management using published guidelines is highly recommended to help identify and treat ILD and/or pneumonitis as effectively and early as possible25,27.
Because of the clinically meaningful impact of T-DXd in HER2-low metastatic breast cancer, additional studies on T-DXd have been conducted or are ongoing. The phase 2 DAISY trial (NCT04132960) included patients classified as having HER2 overexpressing (IHC 3+ or ISH+), HER2-low (IHC 1+ or IHC 2+/ISH−) or HER2 IHC 0 metastatic breast cancer (defined as no staining observed or membrane staining that is incomplete and is faint or barely perceptible and in ≤10% of tumor cells per ASCO/CAP guidelines3). More than 50% of patients were heavily pretreated (five or more previous lines of therapy in the metastatic setting). Antitumor activity by T-DXd was observed at all HER2 expression levels, including in HER2 IHC 0 metastatic breast cancer28. However, low concordance between pathologists in scoring samples as either IHC 0 or IHC 1 was found. Independently, results suggested that ultralow levels of HER2 expression (HER2-ultralow, defined as IHC 0 with faint or barely perceptible membrane staining in ≤10% of tumor cells) could be sufficient to facilitate the uptake of T-DXd, and that drug efficacy could also be partially mediated by HER2-independent mechanisms28. DESTINY-Breast15, a multicenter, open-label, single-arm, phase 3b trial, is currently underway to evaluate the efficacy and safety of T-DXd in patients with HR+ or HR−, HER2-low (IHC 1+ or IHC 2+/ISH−) or HER2 IHC 0 unresectable or metastatic breast cancer in an earlier treatment setting (patients who received up to two prior lines of therapy in the metastatic setting; per clinicaltrials.gov (https://clinicaltrials.gov/study/NCT05950945), last accessed 1 August 2025).
The recently reported DESTINY-Breast06 (NCT04494425) trial evaluated the efficacy of T-DXd in patients with HR+, HER2-low and HER2-ultralow breast cancer who received prior endocrine therapy but not chemotherapy for metastatic disease29. Results showed significant improvement in median PFS by BICR, the primary endpoint, for T-DXd compared with TPC (13.2 versus 8.1 months, respectively; hazard ratio 0.62; P < 0.001) in the HER2-low population. Efficacy results were also consistent between the HER2-low and HER2-ultralow cohorts29. Based on the DESTINY-Breast06 results, T-DXd 5.4 mg kg−1 of body weight has been approved for the treatment of patients with HR+/HER2-low and HR+/HER2-ultralow breast cancer, as determined by a locally or regionally approved test, who have progressed on one or more endocrine therapies in the metastatic setting30.
This is the last updated analysis of DESTINY-Breast04 and confirms the use of T-DXd as the standard of care after chemotherapy in patients with HER2-low metastatic breast cancer. The clinically meaningful benefit and generally manageable safety profile were consistent with the primary analysis8. However, recognition of HER2-low and, more recently, HER2-ultralow breast cancer remains challenging and is an ongoing area of research. In DESTINY-Breast04, identification of HER2-low samples was based on a conventional IHC test. Although high concordance (78%) was observed between historical (local) and centrally assessed patient samples for DESTINY-Breast0431 we acknowledge the difficulties in accurately identifying low levels of HER2 expression and differentiating them from samples that lack HER2 expression (IHC 0 with no staining observed)32,33. Along with the results of the DESTINY-Breast06 trial and the development of other targeted therapies, the treatment landscape for patients with HER2-low and HER2-ultralow breast cancer is expanding, highlighting the need for further assay optimization and training on scoring of HER2 IHC 0 and HER2-ultralow samples for optimal treatment selection and patient care in the future33,34.
Methods
Study design
DESTINY-Breast04 (NCT03734029) is a multicenter, open-label, randomized, phase 3 study designed to compare the efficacy and safety of T-DXd and TPC in patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH−) breast cancer previously treated with one to two lines of chemotherapy in the metastatic setting. Details of the DESTINY-Breast04 study design have been published previously along with the study protocol8.
Eligible patients had centrally confirmed HER2-low breast cancer and had received chemotherapy for metastatic disease or had disease recurrence during or within 6 months after completing adjuvant chemotherapy. Patients with HR+ disease must have received at least one line of endocrine therapy. Patients with treated, stable brain metastases were eligible for the study only if they were asymptomatic and did not require treatment with corticosteroids or anticonvulsants and had recovered from the acute toxic effect of radiotherapy. Patients were excluded from the trial if they had uncontrolled or significant cardiovascular disease or if they had a history of noninfectious ILD that was treated with glucocorticoids or had suspected ILD at screening. No sex- or gender-based analyses were performed because only three male patients were included in the study. Sex was based on self-reported demographics obtained at screening, per protocol.
Patients were randomly assigned in a 2:1 ratio to receive T-DXd or TPC, which included capecitabine, eribulin, gemcitabine, paclitaxel or nab-paclitaxel8. Randomization stratification factors were HER2 IHC/ISH status (IHC 1+ versus IHC 2+/ISH−), number of previous lines of chemotherapy for metastatic disease (1 versus 2), and HR status (positive versus negative).
T-DXd was administered intravenously at a dose of 5.4 mg kg−1 of body weight every 3 weeks. TPC was administered according to the local label or the National Comprehensive Cancer Network guidelines.
Trial oversight
The trial was designed and sponsored by Daiichi Sankyo in collaboration with AstraZeneca and was approved by the institutional review board at each study site. The trial was conducted in adherence with the International Council for Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki, and local regulations on the conduct of clinical research. Written informed consent was provided by all patients before trial participation. Patients did not receive compensation for participating in the study.
IHC scoring
IHC scores for HER2 expression were determined at a central laboratory according to an algorithm adapted from the 2018 ASCO/CAP guidelines, which was previously published3,8. Adequately archived or recent tumor biopsy specimens were assessed using the VENTANA HER2/neu (4B5) assay (Roche Diagnostics), which was for investigational use only at the time of the study. The Roche 4B5 assay is marketed as PATHWAY anti-HER-2/neu (4B5) Rabbit Monoclonal Primary Antibody in the USA (cat. no. 05999570001); as PATHWAY anti-HER-2/neu (4B5) Rabbit Monoclonal Primary Antibody in the ex-US and ex-EU markets under the In Vitro Diagnostic Directive; as VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody in the CE markets under the In Vitro Diagnostic Directive; and as VENTANA HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx in the CE markets under In Vitro Diagnostic Regulation. Please refer to the most updated product labeling for each country. ISH testing was conducted on specimens with central HER2 IHC scores of 2+ using the INFORM HER2 Dual ISH DNA Probe Cocktail investigational use only assay (Roche Diagnostics). For HR status, the tumor was deemed positive for ER and/or PR if ≥1% of tumor cell nuclei were immunoreactive2. The tumor was deemed negative for ER and/or PR if <1% of tumor cell nuclei were immunoreactive2. For the exploratory analysis of the ER expression subgroups, tumors were categorized as ER >10% or ER-low-positive (1%–10%) IHC staining as per ASCO/CAP guidelines2.
Endpoints
The data cutoff for the primary endpoint of PFS by BICR in patients with HR+, HER2-low metastatic breast cancer was planned when approximately 318 PFS events had been observed, and the primary analysis reported all events accrued on or before that data cutoff date. DESTINY-Breast04 achieved statistical significance for T-DXd versus TPC on its primary endpoint as well as key secondary endpoints (PFS by BICR in the overall cohort and OS in the overall and HR+ cohorts) at the time of the primary analysis8. Because the statistical boundary for the primary and secondary endpoints was crossed during the primary analysis, BICR analysis was concluded. For the updated data cutoff (1 March 2023), analyses included OS and PFS by investigator in the overall (HR+ and HR−) and HR+ cohorts. We also conducted exploratory analyses of OS and PFS by investigator assessment in the HR− cohort and ER expression subgroups (IHC 1%–10%; IHC >10%).
For the OS and PFS by investigator endpoints, the HR status was based on data collected with the use of the interactive web-response and voice-response system. The overall and HR+ cohorts included patients whose HR status was misstratified. For the other endpoints and the exploratory analyses of the HR− cohort, HR status was based on the electronic data capture that was corrected for misstratification. Sensitivity analyses performed at the time of the primary analysis showed a limited impact on the results due to the misstratification.
Adverse events were coded and graded according to the Medical Dictionary for Regulatory Activities (v.24.0) and National Cancer Institute Common Terminology Criteria for Adverse Events (v.5.0). An independent adjudication committee evaluated any potential cases of ILD or pneumonitis.
Statistical analyses
This updated OS analysis was performed after an extended median follow-up of 32.0 months (95% CI 31.0–32.8). The data cutoff date (1 March 2023) was for approximately 333 OS events in patients with HR+ disease. For OS and PFS, the median event times were estimated using the Kaplan–Meier method; the Brookmeyer and Crowley method was used to calculate the two-sided 95% CIs for the medians of each treatment arm. A stratified Cox proportional hazards regression model was used to estimate hazard ratios and 95% CIs. The proportional hazard assumption was examined through visual inspection of the graphs of the log of negative log of estimated survival functions. Consistent with the primary analysis, the stratified Cox model included the following stratification factors: HER2 status, number of prior lines of chemotherapy and HR or CDK status8. Efficacy analyses were performed for patients randomly assigned to receive study treatment. Safety analyses were performed in patients who received at least one dose of a trial drug. No imputation was performed for missing or dropout data. In the time-to-event analyses (PFS and OS), patients with missing data were censored. Statistical analyses were conducted using Statistical Analysis System Software v.9.3 or later.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Anonymized individual participant data (IPD) on completed studies and applicable supporting clinical trial documents may be available upon request at https://vivli.org. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study subjects. Details on data-sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/. Additional information can be found in the Supplementary Information.
References
Harbeck, N. et al. Breast cancer. Nat. Rev. Dis. Primers 5, 66–96 (2019).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 147, 993–1000 (2023).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013).
Tarantino, P. et al. HER2-low breast cancer: pathological and clinical landscape. J. Clin. Oncol. 38, 1951–1962 (2020).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J. Clin. Oncol. 38, 1887–1896 (2020).
Tarantino, P. et al. ESMO Expert Consensus Statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann. Oncol. 34, 645–659 (2023).
Ivanova, M. et al. Standardized pathology report for HER2 testing in compliance with 2023 ASCO/CAP updates and 2023 ESMO consensus statements on HER2-low breast cancer. Virchows Arch. 484, 3–14 (2024).
Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7, 1–12 (2021).
Viale, G. et al. Retrospective study to estimate the prevalence and describe the clinicopathological characteristics, treatments received, and outcomes of HER2-low breast cancer. ESMO Open 8, 101615–101625 (2023).
Hwang, K. T. et al. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: a population-based study using SEER database. Clin. Cancer Res. 25, 1970–1979 (2019).
Malainou, C. P. et al. Estrogen-receptor-low-positive breast cancer: pathological and clinical perspectives. Curr. Oncol. 30, 9734–9745 (2023).
Yoder, R. et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer 8, 80–86 (2022).
Chen, T. et al. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin. Breast Cancer 18, 1–8 (2018).
Poon, I. K. et al. The significance of highlighting the oestrogen receptor low category in breast cancer. Br. J. Cancer 123, 1223–1227 (2020).
Schrodi, S. et al. Outcome of breast cancer patients with low hormone receptor positivity: analysis of a 15-year population-based cohort. Ann. Oncol. 32, 1410–1424 (2021).
Nakada, T. et al. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. (Tokyo) 67, 173–185 (2019).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
ENHERTU® (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use, label. Daiichi Sankyo https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761139s028lbl.pdf (2024).
Rakha, E. A. et al. Assessment of predictive biomarkers in breast cancer: challenges and updates. Pathobiology 89, 263–277 (2022).
Saura, C. et al. Pooled analysis by best confirmed response to trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2+ metastatic breast cancer (mBC) from DESTINY-Breast-01, -02, and -03. J. Clin. Oncol. 42(Suppl. 2), S335 (2024).
Rugo, H. S. et al. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open 7, 100553–100564 (2022).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Antiemesis V.2.2025 (National Comprehensive Cancer Network, 2025).
Swain, S. M. et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis—focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 106, 102378–102390 (2022).
Mosele, F. et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat. Med. 29, 2110–2120 (2023).
Bardia, A. et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer. N. Engl. J. Med. 39, 2110–2122 (2024).
ENHERTU® approved in the EU as first HER2 directed therapy for patients with HR positive, HER2 low or HER2 ultralow metastatic breast cancer following at least one endocrine therapy, press release. Daiichi Sankyo https://www.daiichisankyo.com/files/news/pressrelease/pdf/202504/20250404_E.pdf (2025).
Prat, A. et al. Determination of HER2-low status in tumors of patients with unresectable and/or metastatic breast cancer in DESTINY-Breast04. Cancer Res. 83(Suppl. 5), HER2-18 (2023).
Salgado, R. F. et al. Human epidermal growth factor receptor 2 (HER2)-low and HER2-ultralow status determination in tumors of patients (pts) with hormone receptor-positive (HR+) metastatic breast cancer (mBC) in DESTINY-Breast06 (DB-06). Ann. Oncol. 35, S1213–S1214 (2024).
Schildhaus, H.-U. et al. Concordance between the DESTINY-Breast04 clinical trial assay (4B5[CDx]) and other HER2 IHC assays for HER2-low breast cancer in real-world practice: first phase of a large-scale, multicenter global ring study. Cancer Res. 84, 1030 (2024).
Fernandez, A. I. et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 8, 1–4 (2022).
Acknowledgements
We thank the patients and their families for their participation and the study site staff for their contributions. Medical writing support, under the direction of the authors, was provided by E. Wolmarans, L. Carroll and R. Hood of ApotheCom, and was funded by Daiichi Sankyo, Inc. in accordance with Good Publication Practice (GPP) guidelines (http://www.ismpp.org/gpp-2022). This study is sponsored by Daiichi Sankyo Inc. In March 2019, AstraZeneca entered into a global development and commercialization collaboration agreement with Daiichi Sankyo for trastuzumab deruxtecan (T-DXd; DS-8201). The sponsor was involved in study conceptualization, design, data collection, analysis, decision to publish and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.M., C.O.A., L.Y., F.X., Y.C. and D.C. contributed to the conception and/or study design. S.M., C.O.A., L.Y. and D.C. contributed to the development of the study protocol. K.Z., S.-A.I., W.J., H.I., Y.H.P., M.V.L., W.L., J.T., N.T.U., A.P., K.P., H.S.R., T.Y., N.H., S.M., M.D.L., J.-Y.P., X.W., A.G., E.T. and D.C. contributed to data collection and quality control. C.O.A., L.Y., F.X. and Y.C. contributed to data analysis. All authors contributed to data interpretation. All authors were involved in drafting and revising the manuscript, and all authors approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: S.M. has received grants from Genentech, AstraZeneca, Seattle Genetics, Pfizer, Daiichi Sankyo, Duality Bio, D3 Bio, Nuvation Bio and BioNTech; has received consulting fees and/or honoraria from Daiichi Sankyo, Eli Lilly, Genentech, Gilead, Macrogenetics, GlaxoSmithKline, Seattle Genetics, Pfizer, Bristol Myers Squib, Nuvation Bio, D3 Bio, AstraZeneca, BioNTech, Boehringer Ingelheim and Avacta. W.J. has received grants from Daiichi Sankyo and AstraZeneca; has received support for attending meetings and/or travel from AstraZeneca, Eisai, Lilly France, Pfizer, Roche, Chugai Pharma, GlaxoSmithKline, Novartis, Pierre Fabre and Sanofi Aventis; has participated on a data safety monitoring or advisory board for Daiichi Sankyo, AstraZeneca, Eisai, Lilly France, Pfizer, Roche, BMS, Novartis, MSD, Seagen and Gilead. H.I. has received grants from Daiichi Sankyo, AstraZeneca and Chugai; has received consulting fees from Daiichi Sankyo, AstraZeneca, Chugai, Lilly, MSD, Pfizer and Gilead; has received honoraria from Daiichi Sankyo, AstraZeneca, Chugai, Lilly, MSD, Pfizer, Taiho and Kyowa Kirin. Y.H.P. has received grants from AstraZeneca, Pfizer, Novartis, MSD, Roche, Gencurix and GenomeInsight; has received consulting fees from Daiichi Sankyo, AstraZeneca, Pfizer, Roche, Eisai, Gilead, MSD, Novartis and Lilly; has received honoraria from Daiichi Sankyo, AstraZeneca, Pfizer, MSD, Novartis, Roche and Gilead; has received support for attending meetings and/or travel from Gilead and MSD; has participated on data safety monitoring or advisory boards for Daiichi Sankyo, AstraZeneca, Roche, Eisai, Gilead, MSD, Novartis and BeiGene. M.V.L. has received honoraria from Daiichi Sankyo, Roche, Novartis and Pfizer; has received support for attending meetings and/or travel from Roche and Pfizer; has participated on data safety monitoring or advisory boards for Novartis and Roche. J.T. has received grants from Daiichi Sankyo and Eli Lilly; has received consulting fees from Daiichi Sankyo and AstraZeneca; has received honoraria from Daiichi Sankyo, AstraZeneca, Eli Lilly and Kyowa Kirin; has led or had a fiduciary role in the West Japan Oncology Group. N.T.U. has received consulting fees from AstraZeneca, Bayer AG, Bristol Myers Squibb, Carna Biosciences, CytoDyn, Daiichi Sankyo, Eisai Co, Eli Lilly, Genentech, Genomic Health, Gilead Sciences, Lavender Health, OncoCyte Corporation, Pear Bio, Peptilogics, Pfizer, Phoenix Molecular Designs, Preferred Medicine, Carisma Therapeutics, Sysmex Corporation, Takeda and Unitech; has stock in Pear Bio and Phoenix Molecular Designs; has had a speaker or preceptorship role with AstraZeneca, Chugai Roche, Daiichi Sankyo, Kyowa Kirin, Pfizer, Total Health Conferencing and Eli Lilly; has research agreements with AnHeart Therapeutics, Eisai, Gilead Sciences, Phoenix Molecular Designs, Daiichi Sankyo, Puma Biotechnology, Merck, Oncolys BioPharma, OBI Pharma, ChemDiv, Tolero Pharmaceuticals and VITRAC Therapeutics. K.Z. has received grants from Roche; has received honoraria from Daiichi Sankyo, AstraZeneca, MSD, Exact Sciences, Lilly, Pierre Fabre, Gilead, Novartis, Pfizer, Roche and Seagen; has received support for attending meetings and/or traveling from Daiichi Sankyo, AstraZeneca, Gilead, Pfizer, Pierre Fabre and Roche; has participated on data safety monitoring or advisory boards for Daiichi Sankyo, AstraZeneca, Exact Sciences, Gilead, Novartis, Lilly, MSD, Pierre Fabre, and Seagen; is part of the steering committee of an international trial for Daiichi Sankyo and Pierre Fabre; is part of the independent data-monitoring committee for an international trial for Roche. A.P. has received grants from Daiichi Sankyo, AstraZeneca, Boehringer, Novartis, Roche, Pfizer, Lilly and Reveal Genomics; has received consulting fees from Daiichi Sankyo, AstraZeneca, Roche, Pfizer, Novartis, Puma, Peptomyc, Reveal Genomics and Ona Therapeutics; has received honoraria from Daiichi Sankyo, Roche, Pfizer and Novartis; has the following patents: CES WO/2018/096191, ERBB2 WO2018/103834A1 (issued), DNADX (pending), HER2DX (pending) and TNBCDX (pending); has a leadership or fiduciary role in Reveal Genomics; has stock or stock options in Reveal Genomics and Ona Therapeutics; is on the board of directors for Reveal Genomics. K.P. has received grants from Daiichi Sankyo, AstraZeneca, Merck, Novartis, Primeview, GSK, BMS, Boehringer Ingelheim, IQVIA, Health Data Specialists AE, MSD, Roche, PPD, Lilly, Gilead, Genesis, Sanofi and Value Research AE; has received consulting fees from Novartis, Gilead, MSD, Lilly and Primeview; has received honoraria from AstraZeneca, MSD, Novartis, Roche, Gilead, Ipsen, Lilly, Pharmabide and Genesis; has received support for attending meetings and/or travel from AstraZeneca, DEMO, MSD, Novartis, Roche, Gilead and Genesis. H.S.R. has received grants from Daiichi Sankyo, AstraZeneca, F. Hoffmann-La Roche Genentech, Gilead Sciences, Lilly, Merck, Novartis, Pfizer, Pionyr Immunotherapeutics, Sermonix Pharmaceuticals, Stemline Therapeutics and OBI Pharma; has received consulting fees from Daiichi Sankyo, Mylan, Viatris, NAPO and Puma. T.Y. has received grants from Daiichi Sankyo, AstraZeneca, Chugai, Taiho, Nippon Kayaku, Eli Lilly, Pfizer, Seagen, MSD and Kyowa Kirin; has received honoraria from Daiichi Sankyo, AstraZeneca, Chugai, Eisai, Novartis Pharma, Taiho, Nippon Kayaku, Kyowa Kirin, Pfizer and Eli Lilly. N.H. has received consulting fees from Gilead, Roche, Sandoz, Sanofi and Seagen; has received honoraria from Daiichi Sankyo, AstraZeneca, Amgen, EPG Communication, Gilead, Lilly, MEDSCAPE, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Sanofi, Seagen, Springer, Viatris and Zuelligpharma; has participated on a data safety monitoring or advisory board for Roche; has a leadership or fiduciary role in the West German Study Group and the European Society for Medical Oncology. S.-A.I. has received grants from Daiichi Sankyo, AstraZeneca, Eisai, Daewoong Pharm, Pfizer, Roche and Boryung Pharm; has received consulting fees from Daiichi Sankyo, AstraZeneca, Hanmi, Eisai, Lilly, MSD, Idience, Novartis, Pfizer, Roche, GSK and Bertis. M.D.L. has received honoraria from Daiichi Sankyo, Eli Lilly, Novartis, Seagen, Takeda, Roche, Tomalab, Gilead, Genetic, Menarini and Sophos; has received support for attending meetings and/or traveling from Gilead, Novartis, Roche and AstraZeneca; participated on a data safety monitoring or advisory board for Pfizer, Daiichi Sankyo, AstraZeneca, Sanofi, Seagen, Novartis, Ipsen, Roche, Pierre Fabre and GSK. J.-Y.P. has received consulting fees from Daiichi Sankyo, AstraZeneca, MSD and Gilead; has received honoraria from Daiichi Sankyo, AstraZeneca, MSD, Lilly, Pfizer, Novartis, Roche, Exact Science and Gilead; has received support for attending meetings and/or travel from AstraZeneca, Eisai, Roche and Pfizer; has participated in a data safety monitoring or advisory board for Sanofi and Novartis. A.G. has received honoraria from Daiichi Sankyo, AstraZeneca, MSD and Roche; has received support for attending meetings and/or travel from Daiichi Sankyo, AstraZeneca and Gilead; has a leadership or fiduciary role on the advisory boards for AstraZeneca, Seagen and Lilly. E.T. has received honoraria from Daiichi Sankyo, AstraZeneca and Eli Lilly. D.C. has received honoraria from Aptitude Health, Roche, Pfizer, Celldex Therapeutics, Carnall Farrar, San Antonio Breast Cancer Consortium, Highfield Communication, AstraZeneca, Global Commercial Organisation—Egypt, Celgene, Chief Scientists Office, Cancer Research UK, HTA, Samsung Bioepis, Eli Lilly, Costello Medical, prIME Oncology, Novartis, PFS, Merck, Puma, F. Hoffmann-La Roche, Clovis Oncology Breast International Group, Breast Cancer Institute, Daiichi Sankyo, Eisai, Elsevier, European Cancer Organisation, Exact Therapeutics, G1 Therapeutics, Galapagos NV, Genentech Inc., GSK (GlaxoSmithKline), ICON Clinical Research, Prima BioMED, RTI Health Solutions, Seattle Genetics, Byondis, Succinct Medical Communications, Seagen, Sanofi, Sapience Therapeutics, Bexon/Zymeworks Biopharmaceuticals, Make Seconds Count, NexGen and IQVIA. C.O.A., L.Y., F.X. and Y.C. are employed by Daiichi Sankyo and have received stock and other ownership interests from Daiichi Sankyo. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Laura Michel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Subgroup analysis: OS in the (a) overall and (b) HR+ cohorts.
CDK, cyclin-dependent kinase; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hormone receptor; IHC, immunohistochemistry; ISH, in situ hybridization; OS, overall survival; T-DXd, trastuzumab deruxtecan; TPC, treatment of physician’s choice of chemotherapy.
Extended Data Fig. 2 Kaplan-Meier analysis of PFS by investigator in the overall cohort.
CI, confidence interval; HR, hormone receptor; PFS, progression-free survival; T-DXd, trastuzumab deruxtecan; TPC, treatment of physician’s choice of chemotherapy. For the OS and PFS by investigator endpoints for the HR+ and all patient cohorts, the HR status is based on data collected using the interactive web-response and voice-response system at the time of randomization, which includes patients who were misstratified.
Extended Data Fig. 3 Kaplan-Meier analysis of PFS by investigator in (a) HR+ and (b) HR− cohorts.
CI, confidence interval; HR, hormone receptor; PFS, progression-free survival; T-DXd, trastuzumab deruxtecan; TPC, treatment of physician’s choice of chemotherapy. For the OS and PFS by investigator endpoints for the HR+ and all patient cohorts, the HR status is based on data collected using the interactive web-response and voice-response system at the time of randomization, which includes patients who were misstratified.
Supplementary information
Supplementary Information
Supplementary Table 1 and Additional data-sharing information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Modi, S., Jacot, W., Iwata, H. et al. Trastuzumab deruxtecan in HER2-low metastatic breast cancer: long-term survival analysis of the randomized, phase 3 DESTINY-Breast04 trial. Nat Med 31, 4205–4213 (2025). https://doi.org/10.1038/s41591-025-03981-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03981-4