Abstract
Over a lifetime, hematopoietic stem cells (HSCs) adjust their lineage output to support age-aligned physiology. In model organisms, stereotypic waves of hematopoiesis have been observed corresponding to defined age-biased HSC hallmarks. However, how the properties of hematopoietic stem and progenitor cells change over the human lifespan remains unclear. To address this gap, we profiled individual transcriptome states of human hematopoietic stem and progenitor cells spanning gestation, maturation and aging. Here we define the gene expression networks dictating age-specific differentiation of HSCs and the dynamics of fate decisions and lineage priming throughout life. We additionally identifiy and functionally validate a fetal-specific HSC state with robust engraftment and multilineage capacity. Furthermore, we observe that classification of acute myeloid leukemia against defined transcriptional age states demonstrates that utilization of early life transcriptional programs associates with poor prognosis. Overall, we provide a disease-relevant framework for heterochronic orientation of stem cell ontogeny along the real time axis of the human lifespan.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw sequencing data from this study is deposited within the National Institutes of Health Database of Genotypes and Phenotypes (accession phs002750). Processed sequencing data is available from the National Center for Biotechnology Information’s Gene Expression Omnibus (accession GSE189161). A navigable web interface for this dataset is available at https://lifetimecd34.hlilab.org.
Code availability
No new code was generated in this study.
References
Li, Y. et al. Single-cell analysis of neonatal HSC ontogeny reveals gradual and uncoordinated transcriptional reprogramming that begins before birth. Cell Stem Cell 27, 732–747 e737 (2020).
Bian, Z. et al. Deciphering human macrophage development at single-cell resolution. Nature 582, 571–576 (2020).
Buenrostro, J. D. et al. Integrated single-cell analysis maps the continuous regulatory landscape of human hematopoietic differentiation. Cell 173, 1535–1548 e1516 (2018).
Cao, J. et al. A human cell atlas of fetal gene expression. Science 370, eaba7721 (2020).
Han, X. et al. Construction of a human cell landscape at single-cell level. Nature 581, 303–309 (2020).
Lu, Y. et al. Single-cell analysis of human retina identifies evolutionarily conserved and species-specific mechanisms controlling development. Dev. Cell 53, 473–491 e479 (2020).
Park, J. E., Jardine, L., Gottgens, B., Teichmann, S. A. & Haniffa, M. Prenatal development of human immunity. Science 368, 600–603 (2020).
Pellin, D. et al. A comprehensive single cell transcriptional landscape of human hematopoietic progenitors. Nat. Commun. 10, 2395 (2019).
Ranzoni, A. M. et al. Integrative single-cell RNA-seq and ATAC-seq analysis of human developmental hematopoiesis. Cell Stem Cell https://doi.org/10.1016/j.stem.2020.11.015 (2020).
Weng, Q. et al. Single-cell transcriptomics uncovers glial progenitor diversity and cell fate determinants during development and gliomagenesis. Cell Stem Cell 24, 707–723 e708 (2019).
Wang, H. et al. Decoding human megakaryocyte development. Cell Stem Cell 28, 535–549 e538 (2021).
Stras, S. F. et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev. Cell 51, 357–373 e355 (2019).
Copley, M. R. et al. The Lin28b–let-7–Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat. Cell Biol. 15, 916–925 (2013).
Rebel, V. I., Miller, C. L., Eaves, C. J. & Lansdorp, P. M. The repopulation potential of fetal liver hematopoietic stem cells in mice exceeds that of their liver adult bone marrow counterparts. Blood 87, 3500–3507 (1996).
Miller, C. L., Rebel, V. I., Helgason, C. D., Lansdorp, P. M. & Eaves, C. J. Impaired steel factor responsiveness differentially affects the detection and long-term maintenance of fetal liver hematopoietic stem cells in vivo. Blood 89, 1214–1223 (1997).
Beaudin, A. E. et al. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell 19, 768–783 (2016).
Li, H. et al. Efficient CRISPR–Cas9 mediated gene disruption in primary erythroid progenitor cells. Haematologica 101, e216–e219 (2016).
Notta, F. et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351, aab2116 (2016).
Rowe, R. G., Mandelbaum, J., Zon, L. I. & Daley, G. Q. Engineering hematopoietic stem cells: lessons from development. Cell Stem Cell 18, 707–720 (2016).
Popescu, D. M. et al. Decoding human fetal liver haematopoiesis. Nature 574, 365–371 (2019).
van Galen, P. et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell 176, 1265–1281 e1224 (2019).
Velten, L. et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 19, 271–281 (2017).
Triana, S. et al. Single-cell proteo-genomic reference maps of the hematopoietic system enable the purification and massive profiling of precisely defined cell states. Nat. Immunol. 22, 1577–1589 (2021).
Vanuytsel, K. et al. Multi-modal profiling of human fetal liver hematopoietic stem cells reveals the molecular signature of engraftment. Nat. Commun. 13, 1103 (2022).
Granja, J. M. et al. Single-cell multiomic analysis identifies regulatory programs in mixed-phenotype acute leukemia. Nat. Biotechnol. 37, 1458–1465 (2019).
Roy, A. et al. Transitions in lineage specification and gene regulatory networks in hematopoietic stem/progenitor cells over human development. Cell Rep. 36, 109698 (2021).
Zhang, Y. et al. Temporal molecular program of human hematopoietic stem and progenitor cells after birth. Dev. Cell 57, 2745–2760 e2746 (2022).
Calvanese, V. et al. Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature 604, 534–540 (2022).
Jardine, L. et al. Blood and immune development in human fetal bone marrow and down syndrome. Nature 598, 327–331 (2021).
Oetjen, K. A. et al. Human bone marrow assessment by single-cell RNA sequencing, mass cytometry, and flow cytometry. JCI Insight 3, e124928 (2018).
Zhang, F., Wu, Y. & Tian, W. A novel approach to remove the batch effect of single-cell data. Cell Discov. 5, 46 (2019).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Hie, B., Bryson, B. & Berger, B. Efficient integration of heterogeneous single-cell transcriptomes using Scanorama. Nat. Biotechnol. 37, 685–691 (2019).
Lopez, R., Regier, J., Cole, M. B., Jordan, M. I. & Yosef, N. Deep generative modeling for single-cell transcriptomics. Nat. Methods 15, 1053–1058 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 e1821 (2019).
Buttner, M., Miao, Z., Wolf, F. A., Teichmann, S. A. & Theis, F. J. A test metric for assessing single-cell RNA-seq batch correction. Nat. Methods 16, 43–49 (2019).
Luecken, M. D. et al. Benchmarking atlas-level data integration in single-cell genomics. Nat. Methods 19, 41–50 (2022).
MacKinney, A. A. Jr. Effect of aging on the peripheral blood lymphocyte count. J. Gerontol. 33, 213–216 (1978).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
Wolf, F. A. et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 20, 59 (2019).
NCBI. Genes and Disease (NCBI, 1998).
Tan, Y. & Cahan, P. SingleCellNet: a computational tool to classify single cell RNA-seq data across platforms and across species. Cell Syst. 9, 207–213 e202 (2019).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Pang, W. W. et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20017 (2011).
Beerman, I. et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010).
Schiebinger, G. et al. Optimal-transport analysis of single-cell gene expression identifies developmental trajectories in reprogramming. Cell 176, 1517 (2019).
Weinreb, C., Wolock, S., Tusi, B. K., Socolovsky, M. & Klein, A. M. Fundamental limits on dynamic inference from single-cell snapshots. Proc. Natl Acad. Sci. USA 115, E2467–E2476 (2018).
Zhang, S., Afanassiev, A., Greenstreet, L., Matsumoto, T. & Schiebinger, G. Optimal transport analysis reveals trajectories in steady-state systems. PLoS Comput. Biol. 17, e1009466 (2021).
Shahan, R. et al. A single-cell Arabidopsis root atlas reveals developmental trajectories in wild-type and cell identity mutants. Dev. Cell 57, 543–560 e549 (2022).
Kotliar, D. et al. Identifying gene expression programs of cell-type identity and cellular activity with single-cell RNA-seq. eLife 8, e43803 (2019).
Leoni, G. A First Course in Sobolev Spaces Vol. 105 (American Mathematical Society, 2009).
Clarke, M. L. et al. MYB insufficiency disrupts proteostasis in hematopoietic stem cells, leading to age-related neoplasia. Blood 141, 1858–1870 (2023).
Magee, J. A. & Signer, R. A. J. Developmental stage-specific changes in protein synthesis differentially sensitize hematopoietic stem cells and erythroid progenitors to impaired ribosome biogenesis. Stem Cell Rep. 16, 20–28 (2021).
Signer, R. A., Magee, J. A., Salic, A. & Morrison, S. J. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509, 49–54 (2014).
Zhao, J. et al. Human hematopoietic stem cell vulnerability to ferroptosis. Cell 186, 732–747 e716 (2023).
van den Brink, S. C. et al. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat. Methods 14, 935–936 (2017).
Slyper, M. et al. A single-cell and single-nucleus RNA-seq toolbox for fresh and frozen human tumors. Nat. Med. 26, 792–802 (2020).
Lauzurica, P. et al. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood 95, 2312–2320 (2000).
Ziegler, S. F., Ramsdell, F. & Alderson, M. R. The activation antigen CD69. Stem Cells 12, 456–465 (1994).
Bujanover, N. et al. Identification of immune-activated hematopoietic stem cells. Leukemia 32, 2016–2020 (2018).
Ranzoni, A. M. et al. Integrative single-cell RNA-seq and ATAC-seq analysis of human developmental hematopoiesis. Cell Stem Cell 28, 472–487 e477 (2021).
Farlik, M. et al. DNA methylation dynamics of human hematopoietic stem cell differentiation. Cell Stem Cell 19, 808–822 (2016).
Tusi, B. K. et al. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60 (2018).
Witkowski, M. T. et al. Extensive remodeling of the immune microenvironment in B cell acute lymphoblastic leukemia. Cancer Cell 37, 867–882 e812 (2020).
Rowe, R. G. et al. The developmental stage of the hematopoietic niche regulates lineage in MLL-rearranged leukemia. J. Exp. Med. 216, 527–538 (2019).
Tyner, J. W. et al. Functional genomic landscape of acute myeloid leukaemia. Nature 562, 526–531 (2018).
Dohner, H. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129, 424–447 (2017).
Weisberg, S. P. et al. ZFX controls propagation and prevents differentiation of acute T-lymphoblastic and myeloid leukemia. Cell Rep. 6, 528–540 (2014).
Starkova, J. et al. Up-regulation of homeodomain genes, DLX1 and DLX2, by FLT3 signaling. Haematologica 96, 820–828 (2011).
Nagel, S. et al. NKL homeobox gene activities in normal and malignant myeloid cells. PLoS ONE 14, e0226212 (2019).
DeBoever, C. et al. Large-scale profiling reveals the influence of genetic variation on gene expression in human induced pluripotent stem cells. Cell Stem Cell 20, 533–546 e537 (2017).
Karamitros, D. et al. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells. Nat. Immunol. 19, 85–97 (2018).
Zheng, S., Papalexi, E., Butler, A., Stephenson, W. & Satija, R. Molecular transitions in early progenitors during human cord blood hematopoiesis. Mol. Syst. Biol. 14, e8041 (2018).
Adelman, E. R. et al. Aging human hematopoietic stem cells manifest profound epigenetic reprogramming of enhancers that may predispose to leukemia. Cancer Discov. 9, 1080–1101 (2019).
Young, K. et al. Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell 28, 1473–1482 e1477 (2021).
Mitchell, E. et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 606, 343–350 (2022).
Tober, J. et al. Maturation of hematopoietic stem cells from prehematopoietic stem cells is accompanied by up-regulation of PD-L1. J. Exp. Med. 215, 645–659 (2018).
Hu, Y. & Smyth, G. K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 (2009).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337 e324 (2019).
Cahan, P. et al. CellNet: network biology applied to stem cell engineering. Cell 158, 903–915 (2014).
Abugessaisa, I. et al. refTSS: a reference data set for human and mouse transcription start sites. J. Mol. Biol. 431, 2407–2422 (2019).
Acknowledgements
We thank H. Lodish, S. Orkin, C. Weinreb, T. Zou and O. Yilmaz for helpful discussion and J. Ritz and the Pasquarello Tissue Bank for technical assistance. This work was funded by Boston Children’s Hospital institutional funds (G.Q.D. and R.G.R.), the Koch Institute for Integrative Cancer Research, NCI P30CA14051, the Charles W. (1955) and Jennifer C. Johnson Cancer Research Fund, NIDDK K08DK123414 and an American Society of Hematology Scholar Award (H.L.), the Barbara K. Ostrom Bioinformatics Facility and Integrated Genomics and Bioinformatics Core (V.L.B.), NIDDK R01DK100854 and NHLBI U01HL134696 (S.T.C), a Career Award at the Scientific Interface, New Frontiers in Research Exploration Grant, and Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (G.S.), the Coordination for the Improvement of Higher Education Personnel, Brazil, Fundação de Âmparo à Pesquisa e Inovação de Santa Catarina (FAPESC, FAB2019121000026), and the Serrapilheira Institute (R-2111-39726) (E.L.R), a fellowship from the Brazilian National Council for Scientific and Technological Development (M.F.) and NIDDK R01DK134515 and R03DK126729 (R.G.R). The results published here are in part based upon data generated by the TCGA Research Network found at https://www.cancer.gov/tcga.
Author information
Authors and Affiliations
Contributions
H.L. and R.G.R. designed the project and experiments. H.L., K.F., M.T.-Y., V.M., D.W. and R.G.R. performed the experiments. P.C., M.K., J.E., A.A., L.G., G.T., S.Z., V.L.B., E.M., D.L., S.G., A.R., E.L.R. and G.S. developed analytical pipelines. H.L., P.C., M.K., J.E., A.A., L.G., S.Z., V.L.B., E.M., K.L., G.G.C., S.G., E.L.R., G.S. and R.G.R. analyzed the data. H.L., J.W., A.F.C., G.B., G.Q.D., S.T.C. and R.G.R. procured samples, funding and reagents. All authors contributed to the writing and editing of the paper.
Corresponding authors
Ethics declarations
Competing interests
A.R. is a cofounder and equity holder of Celsius Therapeutics, an equity holder in Immunitas and was an SAB member of ThermoFisher Scientific, Syros Pharmaceuticals, Neogene Therapeutics and Asimov. From 1 August 2020, A.R. is an employee of Genentech. From 13 December 2021, J.W. is an employee of Vor Biopharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Anna Bigas, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Madhura Mukhopadhyay, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Persistent batch effect when integrating other published hematopoiesis single cell RNA studies.
(a) Schematic of ages of human samples profiled by nine other human hematopoiesis single cell RNA-sequencing (scRNA-seq) studies with accessible read count data and samples profiled in our current study. Each dot denotes a single point in developmental time captured by each study. The nine combined previously published studies span from four weeks gestation through age 87 and our current study spans from 10 weeks’ gestation through age 77. (b) Assessment of integrated scRNA-seq datasets obtained by batch correction using fastMNN, Harmony, Scanorama, scVI, or Seurat. Displayed are a uniform manifold approximation and projection (UMAP), k-nearest neighbor batch effect test (kBET), and lifetime dynamics of lymphoid contribution to total hematopoiesis for the datasets generated using each of the five batch correction methods. For UMAP panels, Louvain clustering was performed, and progenitor cell types were identified using marker gene analysis and SingleCellNet random forest classification against hematopoietic lineage cell type profiles21. For kBET panels, box and whisker plots denote kBET rejection rate, where a lower rejection rate indicates fewer persistent batch effects (that is replicates are well-mixed). The ideal/expected value represents the expected rejection rate if batch correction ideally mixes the collection of integrated studies, while the observed value represents the rejection rate after employing the indicated batch-correction method. For lymphoid contribution to hematopoiesis panels, for each dataset resulting from the indicated batch correction methods, the fraction of hematopoietic progenitor cells identified as lymphoid progenitor cells was identified for each sample and a LOESS fit was applied to generate a curve over the time course of all samples. Note that none of the five methods yield a batch-corrected dataset where there is a spike in lymphoid contribution to hematopoiesis during childhood.
Extended Data Fig. 2 Isolation and scRNA-seq quality control of human CD34+ HSPCs.
(a) Representative sorting scheme for isolation of CD34+ HSPCs from fetal liver (FL), umbilical cord blood (CB) and postnatal bone marrow (BM) specimens. (b) Summary of scRNA-seq quality control data for each donor/library included in this study indicating unique genes per single cell (top), unique molecular identifiers (UMIs) per cell (middle), and percentage mitochondrial reads (bottom). (c) Single cells from each of the indicated donors are highlighted in red and projected within UMAP space for the entire dataset. (d) Expression levels of fetal-associated genes IGF2BP3, HMGA2, and LIN28B are displayed as violin plots for all cells within the seven age ranges of human life profiled in this study. The horizontal line in each violin plot indicates mean expression level. Significance of all pairwise comparisons between age ranges is also displayed. All p-values were calculated using the two-sided Wilcoxon rank-sum test followed by Bonferroni correction for multiple (28 total) comparisons. All adjusted p-values >0.05 are in shades of blue, =0.05 in white, and <0.05 in shades of red. (e) The percent of X and Y chromosome genes present in each of the indicated gene sets and top 50 contributing genes to each of the indicated gene sets is displayed. Marker genes for each age range were identified through differential expression of all cells from that age range versus the rest of the full dataset. (f) kBET analysis yields no statistically significant difference (p-value >0.05 using the kBet implementation of Pearson’s χ2 test across independent library preparations of 2 groups of donors) between Ideal/Expected rejection rate for our dataset and the rejection rate of Seurat batch correction of our human lifetime dataset. Rejection rate and 95% confidence interval for rejection rate are indicated. Additionally, note that in our dataset there is a peak in lymphoid progenitor cell contribution to total hematopoiesis during childhood.
Extended Data Fig. 3 Transcriptome-based cell type annotation of human CD34+ HSPCs.
(a) Unsupervised Louvain clustering was used to identify 22 transcriptionally distinct HSPC clusters which were annotated based on marker gene expression and position in UMAP space. For lineages with multiple progenitor clusters, lower number designation (for example, E-Prog-1) indicates less apparent differentiation and a higher number (for example, E-Prog-3) indicates an overall greater degree of commitment to that lineage. Clusters are organized into lineage groups based on priming towards a single lineage or based on multipotency. (b) singleCellNet classification of HSPC states against a healthy human adult bone marrow scRNA-seq dataset21. (c) The five lymphoid progenitor clusters making up the two lymphoid trajectories in UMAP space are analyzed for cell cycle status based on gene expression signatures. Upon cell cycle regression of the dataset, the two lymphoid trajectories integrate into a single lymphoid trajectory in UMAP space. (d) Potential doublets were removed from the dataset using DoubletFinder82 and UMAP was performed on remaining cells. The relationship of lineages and their trajectories is similar comparing the full dataset (top UMAP) and dataset upon removal of potential doublets (bottom UMAP). Color scheme for lineages is the same as in panel C. (e) CD34+ HSPCs were isolated at the indicated ages and cultured in methylcellulose medium with cytokines for 14 days, at which time colony frequency and morphology was tabulated in a blinded fashion. * indicates samples where scRNA-seq was also performed. CFU-GEMM = colony forming unit granulocyte/erythroid/monocyte/megakaryocyte BFU-E = burst forming unit erythroid CFU-GM = colony forming unit granulocyte/monocyte CFU-M = colony forming unit monocyte CFU-G = colony forming unit granulocyte.
Extended Data Fig. 4 Population balance analysis (PBA) and stationary optimal transport (StatOT) fate probability prediction.
Diffusion-drift modeling was used to define commitment probabilities to lineage fates for HSPCs by PBA and StatOT. (a-b) We compare the proportion of mass (as measured by fraction of total predicted hematopoietic output) by lineage and age in terminal fates for (a) PBA and (b) StatOT to the proportion of cells that reside in annotated clusters of differentiated cell types (observed probability mass). The proportion of mass derived from PBA and StatOT represent the expected proportion of cells ending up with a particular lineage fate, though individual cells may have the potential to acquire multiple fates. Agreement between observed and expected probabilities were used as a metric for evaluating performance of the probabilistic algorithms. (c) Schematic of triangle fate probability plots that serve as output from diffusion-drift modeling. Individual cell fate lineage probability is defined based on location relative to lymphoid, myeloid (including monocyte), and erythro-megakaryocytic fates. Centroid (*) indicates the expected average HSPC probability. Also displayed is color scheme for individual cells, which is based on cell type cluster category from Louvain clustering (Extended Data Fig. 3a), and is used for the remainder of Extended Data Fig. 4. (d) Individual cell lineage commitment probabilities to lymphoid, myeloid, and erythro-megakaryocytic fates for HSPCs in each age phase by population balance analysis (PBA) and stationary optimal transport (StatOT). (e) Individual cell lineage commitment probabilities for HSPCs in all 26 donors in the dataset. (f) Focusing only on the least differentiated, hematopoietic stem cell (HSC)-containing state cluster, the mean PBA and StatOT probabilities of bias towards myeloid, erythro-megakaryocytic, or lymphoid fates at each age are displayed. (g) Upon comparing the lineage fate probabilities between two age ranges, the significance of each difference was calculated using Student’s t-test, with p-value for each pair-wise comparison displayed as a heatmap.
Extended Data Fig. 5 Consensus non-negative matrix factorization (cNMF) and gene expression program (GEP) quality control.
(a) Stability and error values for cNMF trials using the indicated number of component gene expression programs (k) noting that 35 component programs were used in the final analysis. (b) Correlation matrix comparing Euclidean distance between the 35 component GEPs indicating the independence of the 35 programs from each other. (c) Spearman coefficient correlation matrix comparing the 35 GEPs. Note that the highest Spearman coefficient value is only 0.59, orthogonally indicating the independence of the 35 GEPs from one another. (d) Gene set enrichment analysis for the indicated lineage signatures against the identified lineage GEPs. Colored shading corresponds with nominal enrichment score (NES) and circle size with false discovery rate (FDR) q value. (e) Normalized average GEP usage per cell was mapped for each sample across the human lifespan, with LOESS fit curves indicating average utilization.
Extended Data Fig. 6 Application of GEP usage to lineage branching and HSC lineage dominance.
(a) Based on the pair-wise comparisons between the six lineage GEPs displayed in Fig. 2a, volcano plots of the negative-log of Bonferroni corrected p-value of the Pearson Correlation coefficient versus the Correlation coefficient itself are displayed for HSCs, MPPs, and Progenitors in all age groups. (b) For fetal, adolescent, and adult MPPs and progenitors identified in26, two-sided Pearson Correlation coefficients and Bonferroni corrected p-values of the coefficient were calculated for all pair-wise comparisons between the six lineage GEPs. These adjusted p-values were then compared to the adjusted p-values of the lineage anti-correlations found to be significant in this current study. (c) A schema is presented describing two methods (division- and subtraction-based) for calculating unilineage dominance score using either lineage GEP usage from single cell transcriptome data or lineage output in clonal outgrowth assays in culture. (d,e) Using a (d) division-based or (e) subtraction-based methodology, unilineage dominance scores were calculated in individual fetal, adult, and elderly HSCs using either lineage GEP expression or lineage output in a clonal outgrowth assay supporting lymphoid, monocyte/dendritic cell, granulocyte, erythroid, and megakaryocyte differentiation. The median unilineage dominance scores (indicated by horizontal lines on the violin plots) from GEP usage and clonal outgrowth for each age were then compared and lineage regressions were performed on the relationship between scores calculated with GEP usage or clonal outgrowth data. Displayed coefficients of determination indicate that for HSCs, unilineage dominance scores calculated from GEP usage and clonal outgrowth data more closely reflect each other when using a division-based methodology. (f) Using both the division-based and subtraction-based methods for calculating unilineage dominance, scores for individual HSCs and individual progenitors were calculated, with median indicated by the horizontal line on each violin plot. Significance testing for the difference between HSCs and progenitors in both the division-based and subtraction-based plots yielded p-value<2.2x10-16 by two-sided Wilcoxon Rank-Sum testing. The increased unilineage dominance score in progenitors compared to HSCs indicates the subtraction-based methodology is preferred for determining magnitude of lineage commitment.
Extended Data Fig. 7 A reproducible fetal HSC gene expression state identified by cNMF.
(a) Viable, lineage- cells from mouse fetal liver and adult bone marrow were stained with antibodies against the indicated surface markers to identify hematopoietic progenitors (c-Kit+ Sca-1+) and HSCs (c-Kit+ Sca-1+ Flk2- CD34-). Both populations from both organ sites were then assessed for cell surface CD69 expression. Percent of parent population values are shown. (b) The percent of CD69+ cells in mouse fetal liver and bone marrow are compared for various HSPC immunophenotypes (gating strategy shown in a). Fetal livers from nine different fetal mice and bone marrow from six different adult mice (biologic replicates) were analyzed. Student’s two-tailed t-test was used to compare percent CD69+ cells in fetal liver and bone marrow, with * indicating p-value <0.05 and **** indicating p-value <0.0001; data are presented as mean +/- SD – p values are LSK 1.1E-8, HSC 0.03, MPP2 4E-8, MPP3 1E-9, MPP4 2E-10. (c) The indicated scRNA-seq datasets were analyzed by classifying cells based on both expression of CD69 and usage of the fetal-HSC-GEP. Enrichment of fetal-HSC-GEP usage was quantified and compared between CD69+ and CD69- fractions, with p-values from two-sided Wilcoxon rank-sum testing and box plots indicating average and +/− 1 standard deviation. (d) In an independent scRNA-seq dataset26 that contains human CD34+ cells from fetal liver and postnatal bone marrow up to early adulthood, a similar analysis as in (c) was performed demonstrating enrichment of fetal-HSC-GEP usage in fetal liver compared to early postnatal bone marrow, with p-values from two-sided Wilcoxon rank-sum and box plots indicating average and +/− 1 standard deviation. (e) In an independent scRNA-seq dataset9 that profiles human fetal liver and fetal bone marrow CD34+ cells, a similar analysis as in (c) was performed demonstrating no significant difference in usage of the fetal-HSC-GEP when comparing fetal liver and fetal bone marrow hematopoiesis, with p-values from two-sided Wilcoxon rank-sum testing. (f) In an independent scRNA-seq dataset64 that captures mouse fetal liver and postnatal bone marrow c-Kit+ cells, a similar analysis as in (c) was performed demonstrating enrichment of fetal-HSC-GEP usage in mouse E13.5 fetal liver compared to adult mouse bone marrow, with p-values from two-sided Wilcoxon rank-sum testing. (g) Using fetal HSC and postnatal HSC DNA methylation data63, the difference in methylation scoring surrounding the transcriptional start site of the top 200 genes contributing to the fetal-HSC-GEP is displayed, indicating greater DNA methylation postnatally, with p-values from two-sided Wilcoxon rank-sum testing.
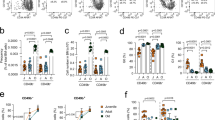
Extended Data Fig. 8 Flow cytometric analysis of CD69+ human HSPCs before and after xenotransplantation engraftment.
(a) Human CD34+ HSPCs were isolated from the indicated tissues including 20-week gestation fetal liver (20 week) with surface CD69 expression analyzed by flow cytometry at initial thawing (with cells having been frozen at the time of isolation) compared to following 48 hours in culture with the indicated cytokines. Mean fluorescence intensities are shown on each graph. (b) Lineage- CD34+ CD38- human fetal liver HSPCs expressing or not expressing surface CD69 (based on lowest 10th percentile and highest 10th percentile expression) were isolated by fluorescence cell sorting, with a representative post-sort purity analysis shown. (c) Bone marrow mononuclear cells were harvested from NOD.Cg-Prkdcscid Il2rgtm1Wj/SzJ (NSG) mice 12 weeks after xenotransplantation with human CD34+ CD38- HSPCs. Representative flow cytometric analysis of human cell engraftment is displayed. Percent of parent population values are shown.
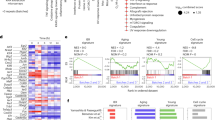
Extended Data Fig. 9 Correlation of HSPC states with acute leukemia scRNA-seq and assessment of age state prognostic independence in acute myeloid leukemia (AML).
(a) scRNA-seq datasets of human B lymphoblastic ALL65 and AML21 were classified against the 22 HSPC states using singleCellNet with classification scores for each state indicated. Only cells annotated as expressing CD19 are included in the ALL analysis and only cells annotated as bearing AML-associated somatic mutations are included in the AML analysis. (b) Cox multivariate hazard ratio analysis comparing age composite score as an independent variable to either age at diagnosis, bone marrow blast percentage, FAB classification, ELN 2017 favorable risk designation, or mutational status of CEBPA, FLT3-ITD, IDH, NPM1, RAS, TP53, and DNMT3A in the BeatAML dataset68. Hazard ratios and 95% confidence intervals for hazard ratios are indicated. (c) Comparison of mixed age scores for AMLs that were preceded by MDS or not preceded by MDS in the BeatAML dataset. The points representing individual AMLs are color-coded by whether they are in the high or low age score group. Significance was calculated using two-sided Wilcoxon rank-sum testing. (d) Overall survival probability in the TCGA dataset for high and low AML age score groups was compared with p-value shown. (e) Overall survival probability in the TCGA dataset for high and low TF score groups was compared with p-value shown. All survival curve p-values were calculated by log-rank test.
Supplementary information
Supplementary Data Table 1
(A) Z-scores for contribution of all genes to all GEPs. (B) Usage level of all GEPs for all cells in this study. (C) Marker gene analysis for the combination of all lineage-specific clusters. The erythroid cluster is defined as E-Prog 1–3, the granulocyte cluster is defined as G/M-Prog and G-Prog-1–3, the lymphoid cluster is defined as Ly-Prog-1–5, the monocyte/dendritic cell cluster is defined as pDC-Prog and Mono/cDC-Prog and the basophil/mast cell (Baso/Mast) and Meg clusters are defined as the single cluster for each. (D) The lineage outcomes for individual colonies from adult and elderly Lineage−CD34+CD38+ progenitor cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Côté, P., Kuoch, M. et al. The dynamics of hematopoiesis over the human lifespan. Nat Methods 22, 422–434 (2025). https://doi.org/10.1038/s41592-024-02495-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41592-024-02495-0
This article is cited by
-
An inflammatory and quiescent HSC subpopulation expands with age in humans
Genome Biology (2026)
-
Aging modulates the immunosuppressive, polarizing and metabolic functions of blood-derived myeloid-derived suppressor cells (MDSCs)
Immunity & Ageing (2025)
-
Systemic deficits in lipid homeostasis promote aging-associated impairments in B cell progenitor development
GeroScience (2025)