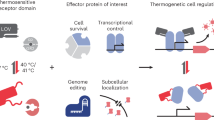
Abstract
Inducible protein switches are currently limited for use in tissues and organisms because common inducers cannot be controlled with precision in space and time in optically dense settings. Here, we introduce a protein that can be reversibly toggled with a small change in temperature, a stimulus that is both penetrant and dynamic. This protein, called Melt (Membrane localization using temperature) oligomerizes and translocates to the plasma membrane when temperature is lowered. We generated a library of Melt variants with switching temperatures ranging from 30 °C to 40 °C, including two that operate at and above 37 °C. Melt was a highly modular actuator of cell function, permitting thermal control over diverse processes including signaling, proteolysis, nuclear shuttling, cytoskeletal rearrangements and cell death. Finally, Melt permitted thermal control of cell death in a mouse model of human cancer. Melt represents a versatile thermogenetic module for straightforward, non-invasive and spatiotemporally defined control of mammalian cells with broad potential for biotechnology and biomedicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All unique biological materials and raw images are available upon request. Source data are provided with this paper.
Code availability
Custom scripts used to analyze data are available upon request.
References
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010).
Ash, C., Dubec, M., Donne, K. & Bashford, T. Effect of wavelength and beam width on penetration in light–tissue interaction using computational methods. Lasers Med. Sci. 32, 1909–1918 (2017).
Piraner, D. I. et al. Going deeper: biomolecular tools for acoustic and magnetic imaging and control of cellular function. Biochemistry 56, 5202–5209 (2017).
Miller, I. C. et al. Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control. Nat. Biomed. Eng. 5, 1348–1359 (2021).
Ermakova, Y. G. et al. Thermogenetic control of Ca2+ levels in cells and tissues. Preprint at bioRxiv https://doi.org/10.1101/2023.03.22.533774 (2023).
Corbett, D. C. et al. Thermofluidic heat exchangers for actuation of transcription in artificial tissues. Sci. Adv. 6, eabb9062 (2020).
Walton, M., Roestenburg, M., Hallwright, S. & Sutherland, J. C. Effects of ice packs on tissue temperatures at various depths before and after quadriceps hematoma: studies using sheep. J. Orthop. Sports Phys. Ther. 8, 294–300 (1986).
ter Haar, G. & Coussios, C. High intensity focused ultrasound: physical principles and devices. Int. J. Hyperthermia 23, 89–104 (2007).
Horowitz, N. H. Biochemical genetics of Neurospora. Adv. Genet. 3, 33–71 (1950).
Talavera, A. & Basilico, C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J. Cell. Physiol. 92, 425–436 (1977).
Varadarajan, R., Nagarajaram, H. A. & Ramakrishnan, C. A procedure for the prediction of temperature-sensitive mutants of a globular protein based solely on the amino acid sequence. Proc. Natl Acad. Sci. USA 93, 13908–13913 (1996).
Hurme, R., Berndt, K. D., Normark, S. J. & Rhen, M. A proteinaceous gene regulatory thermometer in Salmonella. Cell 90, 55–64 (1997).
Piraner, D. I., Wu, Y. & Shapiro, M. G. Modular thermal control of protein dimerization. ACS Synth. Biol. 8, 2256–2262 (2019).
Guo, Y., Liu, S., Jing, D., Liu, N. & Luo, X. The construction of elastin-like polypeptides and their applications in drug delivery system and tissue repair. J. Nanobiotechnology 21, 418 (2023).
Despanie, J., Dhandhukia, J. P., Hamm-Alvarez, S. F. & MacKay, J. A. Elastin-like polypeptides: therapeutic applications for an emerging class of nanomedicines. J. Control. Release 240, 93–108 (2016).
Li, Z., Tyrpak, D. R., Park, M., Okamoto, C. T. & MacKay, J. A. A new temperature-dependent strategy to modulate the epidermal growth factor receptor. Biomaterials 183, 319–330 (2018).
Abedi, M. H., Lee, J., Piraner, D. I. & Shapiro, M. G. Thermal control of engineered T-cells. ACS Synth. Biol. 9, 1941–1950 (2020).
Wu, Y. et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 5, 1336–1347 (2021).
Morimoto, R. I. Cells in stress: transcriptional activation of heat shock genes. Science 259, 1409–1410 (1993).
Feder, M. E. & Hofmann, G. E. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282 (1999).
Akerfelt, M., Morimoto, R. I. & Sistonen, L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Benman, W. et al. Temperature-responsive optogenetic probes of cell signaling. Nat. Chem. Biol. 18, 152–160 (2022).
Glantz, S. T. et al. Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids. Proc. Natl Acad. Sci. USA 115, E7720–E7727 (2018).
Benman, W., Iyengar, P., Mumford, T., Huang, Z. & Bugaj, L. J. Multiplexed dynamic control of temperature to probe and observe mammalian cells. Preprint at bioRxiv https://doi.org/10.1101/2024.02.18.580877 (2024)
Pal, A. A. et al. Optogenetic clustering and membrane translocation of the BcLOV4 photoreceptor. Proc. Natl Acad. Sci. USA 120, e2221615120 (2023).
Harper, S. M., Neil, L. C. & Gardner, K. H. Structural basis of a phototropin light switch. Science 301, 1541–1544 (2003).
Huang, Z., Benman, W., Dong, L. & Bugaj, L. Rapid optogenetic clustering in the cytoplasm with BcLOVclust. J. Mol. Biol. 436, 168452 (2024).
Mumford, T. R. et al. Simple visualization of submicroscopic protein clusters with a phase-separation-based fluorescent reporter. Cell Syst. 15, 166–179 (2024).
Grecco, H. E., Schmick, M. & Bastiaens, P. I. H. Signaling from the living plasma membrane. Cell 144, 897–909 (2011).
Toettcher, J. E., Weiner, O. D. & Lim, W. A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).
Citri, A. & Yarden, Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 (2006).
Liang, S. I. et al. Phosphorylated EGFR dimers are not sufficient to activate Ras. Cell Rep. 22, 2593–2600 (2018).
Chung, H. K. et al. A compact synthetic pathway rewires cancer signaling to therapeutic effector release. Science 364, eaat6982 (2019).
Gao, X. J., Chong, L. S., Kim, M. S. & Elowitz, M. B. Programmable protein circuits in living cells. Science 361, 1252–1258 (2018).
Sanchez, M. I. & Ting, A. Y. Directed evolution improves the catalytic efficiency of TEV protease. Nat. Methods 17, 167–174 (2020).
Zhang, Q. et al. Designing a green fluorogenic protease reporter by flipping a beta strand of GFP for imaging apoptosis in animals. J. Am. Chem. Soc. 141, 4526–4530 (2019).
Collas, P. & Aleström, P. Nuclear localization signal of SV40 T antigen directs import of plasmid DNA into sea urchin male pronuclei in vitro. Mol. Reprod. Dev. 45, 431–438 (1996).
Dorfman, J. & Macara, I. G. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol. Biol. Cell 19, 1614–1626 (2008).
Heo, W. D. et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006).
He, L. et al. Optical control of membrane tethering and interorganellar communication at nanoscales. Chem. Sci. 8, 5275–5281 (2017).
Regot, S., Hughey, J. J., Bajar, B. T., Carrasco, S. & Covert, M. W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–1734 (2014).
Levskaya, A., Weiner, O. D., Lim, W. A. & Voigt, C. A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Berlew, E. E. et al. Designing single-component optogenetic membrane recruitment systems: the Rho-family GTPase signaling toolbox. ACS Synth. Biol. 11, 515–521 (2022).
Hannanta-Anan, P., Glantz, S. T. & Chow, B. Y. Optically inducible membrane recruitment and signaling systems. Curr. Opin. Struct. Biol. 57, 84–92 (2019).
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Shkarina, K. et al. Optogenetic activators of apoptosis, necroptosis, and pyroptosis. J. Cell Biol. 221, 202109038 (2022).
Ntombela, L., Adeleye, B. & Chetty, N. Low-cost fabrication of optical tissue phantoms for use in biomedical imaging. Heliyon 6, e03602 (2020).
Gallouzi, I. E. et al. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc. Natl Acad. Sci. USA 97, 3073–3078 (2000).
Kedersha, N. L., Gupta, M., Li, W., Miller, I. & Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442 (1999).
Berlew, E. E. et al. Single-component optogenetic tools for inducible RhoA GTPase signaling. Adv. Biol. 5, e2100810 (2021).
Berlew, E. E., Kuznetsov, I. A., Yamada, K., Bugaj, L. J. & Chow, B. Y. Optogenetic Rac1 engineered from membrane lipid-binding RGS-LOV for inducible lamellipodia formation. Photochem. Photobiol. Sci. 19, 353–361 (2020).
Qiao, J., Peng, H. & Dong, B. Development and application of an optogenetic manipulation system to suppress actomyosin activity in Ciona epidermis. Int. J. Mol. Sci. 24, 5707 (2023).
Wang, W. et al. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat. Biotechnol. 35, 864–871 (2017).
Tidyr. https://tidyr.tidyverse.org/
Wickham, H. ggplot2. WIREs Comput. Stat. 3, 180–185 (2011).
Legland, D., Arganda-Carreras, I. & Andrey, P. MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32, 3532–3534 (2016).
Bugaj, L. J. & Lim, W. A. High-throughput multicolor optogenetics in microwell plates. Nat. Protoc. 14, 2205–2228 (2019).
Acknowledgements
The authors thank E. Berlew and B. Chow for helpful discussions on BcLOV4 activity and for plasmids encoding BcLOV(Q355N) and BcLOV-ITSN1; and A. Hughes and M. Good for helpful comments on the manuscript. The authors also thank the Penn Cytomics and Cell Sorting Shared Resource Laboratory for assistance with cell sorting. This work was supported by funding from the National Institutes of Health (R35GM138211 for L.J.B.), the National Science Foundation (Graduate Research Fellowship Program to W.B., CAREER 2145699 to L.J.B.), and the Penn Center for Precision Engineering for Health (CPE4H). Cell sorting was performed on a BD FACSAria Fusion that was obtained through NIH S10 1S10OD026986.
Author information
Authors and Affiliations
Contributions
W.B. and L.J.B. conceived the study. W.B. generated Melt and its integration into molecular circuits. Z.H. discovered and characterized thermostable Melt variants, which were then integrated into circuits by Z.H. and W.B. W.B. and P.I. developed and validated the thermoPlate. D.W. and T.R.M. validated cluster-induced cell killing. W.B., Z.H. and P.I. performed and analyzed all experiments. L.J.B. supervised the work. W.B., Z.H. and L.J.B. wrote the manuscript and produced the figures. All of the authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks G. Woolley, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Information
Supplementary Video 1
Reversible membrane binding of Melt using temperature.
Supplementary Video 2
Temperature-controlled nucleocytoplasmic shuttling of MeltNLS/NES.
Supplementary Video 3
Thermal control of Erk activity in mammalian temperature ranges using MeltEGFR-37.
Supplementary Video 4
Temperature-controlled nucleocytoplasmic shuttling of MeltNLS/NES-40 in mammalian temperature ranges.
Supplementary Video 5
Reversible changes in cell size through thermal control of MeltITSN1-37.
Supplementary Video 6
Temperature-inducible cell death using MeltCasp1-37.
Source data
Source Data Figs. 1–6
Source data for all plots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benman, W., Huang, Z., Iyengar, P. et al. A temperature-inducible protein module for control of mammalian cell fate. Nat Methods 22, 539–549 (2025). https://doi.org/10.1038/s41592-024-02572-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41592-024-02572-4