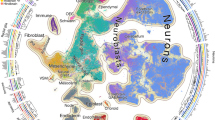
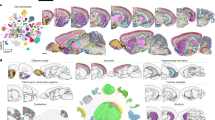
Abstract
Development of the mammalian brain requires precise molecular changes across diverse cell lineages. While single-cell RNA abundances in the developing brain have been characterized by single-cell RNA sequencing (scRNA-seq), single-cell protein abundances have not been characterized. To address this gap, we performed mass cytometry on the whole brain at embryonic day (E)11.5–E12.5 and the telencephalon, the diencephalon, the mesencephalon and the rhombencephalon at E13.5–postnatal day (P)4 from C57/BL6 mice. Using a 40-antibody panel to analyze 24,290,787 cells from two to four biological replicates per sample, we identify 85 molecularly distinct cell clusters from distinct lineages. Our analyses confirm canonical molecular pathways of neurogenesis and gliogenesis, and predict two distinct trajectories for cortical oligodendrogenesis. Differences in protein versus RNA expression from mass cytometry and scRNA-seq, validated by immunohistochemistry and RNAscope in situ hybridization (ISH), demonstrate the value of protein-level measurements for identifying functional cell states. Our findings show the utility of mass cytometry as a scalable platform for single-cell profiling of brain tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Requests for reagents and other resources will be fulfilled by E.R.Z. (ezunder@virginia.edu). Raw single-cell mass cytometry FCS files have been deposited at FlowRepository (https://flowrepository.org/) under accession number FR-FCM-Z7CQ. FCS files for each individual sample (debarcoded, normalized and batch corrected) and clean-up gating used for sample preprocessing are available on Cytobank (https://cytobank.org/cytobank/experiments/) under experiment IDs 105280–105284. The scRNA-seq dataset from La Manno et al.42 is available at the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under accession PRJNA637987. Source data are provided with this paper.
Code availability
Code used to perform analysis of mass cytometry data (as detailed in the Methods) was adapted from standard R and Python packages (for example, UMAP version 0.5.3, LeidenAlg version 0.8.2, Seurat version 4.3.0, URD version 1.1.1 and ggplot2 version 3.4.0) and is available on GitHub at https://github.com/zunderlab/VanDeusen-et-al.-CNS-Development-Manuscript. More detailed information is available upon request.
References
Yuste, R. et al. A community-based transcriptomics classification and nomenclature of neocortical cell types. Nat. Neurosci. 23, 1456–1468 (2020).
La Manno, G. et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580 (2016).
Kee, N. et al. Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem Cell 20, 29–40 (2017).
Frazer, S. et al. Transcriptomic and anatomic parcellation of 5-HT3AR expressing cortical interneuron subtypes revealed by single-cell RNA sequencing. Nat. Commun. 8, 14219 (2017).
Chen, Y.-J. J. et al. Single-cell RNA sequencing identifies distinct mouse medial ganglionic eminence cell types. Sci. Rep. 7, 45656 (2017).
Yuzwa, S. A. et al. Developmental emergence of adult neural stem cells as revealed by single-cell transcriptional profiling. Cell Rep. 21, 3970–3986 (2017).
Mayer, C. et al. Developmental diversification of cortical inhibitory interneurons. Nature 555, 457–462 (2018).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Tiklová, K. et al. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 10, 581 (2019).
Wizeman, J. W., Guo, Q., Wilion, E. M. & Li, J. Y. Specification of diverse cell types during early neurogenesis of the mouse cerebellum. eLife 8, e42388 (2019).
Romanov, R. A. et al. Molecular design of hypothalamus development. Nature 582, 246–252 (2020).
Zhou, X. et al. Cellular and molecular properties of neural progenitors in the developing mammalian hypothalamus. Nat. Commun. 11, 4063 (2020).
Kim, D. W. et al. The cellular and molecular landscape of hypothalamic patterning and differentiation from embryonic to late postnatal development. Nat. Commun. 11, 4360 (2020).
Lee, D. R. et al. Transcriptional heterogeneity of ventricular zone cells in the ganglionic eminences of the mouse forebrain. eLife 11, e71864 (2022).
Bandura, D. R. et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Leelatian, N. et al. Single cell analysis of human tissues and solid tumors with mass cytometry. Cytometry B Clin. Cytom. 92, 68–78 (2017).
Hu, A. X. et al. in Brain Tumor Stem Cells: Methods and Protocols (eds Singh, S. K. & Venugopal, C.) 155–168 (Springer, 2019).
Leelatian, N. et al. Unsupervised machine learning reveals risk stratifying glioblastoma tumor cells. eLife 9, e56879 (2020).
Galdieri, L. et al. Defining phenotypic and functional heterogeneity of glioblastoma stem cells by mass cytometry. JCI Insight 6, 128456 (2021).
Ajami, B. et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551 (2018).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Böttcher, C. et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 22, 78–90 (2019).
Böttcher, C. et al. Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl. Psychiatry 10, 310 (2020).
Li, S. et al. Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. J. Neuroinflammation 18, 7 (2021).
Xie, M. et al. TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration. Nat. Neurosci. 25, 26–38 (2022).
Keeler, A. B. et al. A developmental atlas of somatosensory diversification and maturation in the dorsal root ganglia by single-cell mass cytometry. Nat. Neurosci. 25, 1543–1558 (2022).
Ogrodnik, M. et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061–1077 (2019).
Liu, Y., Beyer, A. & Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550 (2016).
Lombard-Banek, C., Moody, S. A. & Nemes, P. Single-cell mass spectrometry for discovery proteomics: quantifying translational cell heterogeneity in the 16-cell frog (Xenopus) embryo. Angew. Chem. 128, 2500–2504 (2016).
Budnik, B., Levy, E., Harmange, G. & Slavov, N. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 19, 161 (2018).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Mimitou, E. P. et al. Scalable, multimodal profiling of chromatin accessibility, gene expression and protein levels in single cells. Nat. Biotechnol. 39, 1246–1258 (2021).
Chen, V. S. et al. Histology atlas of the developing prenatal and postnatal mouse central nervous system, with emphasis on prenatal days E7.5 to E18.5. Toxicol. Pathol. 45, 705–744 (2017).
Mi, D. et al. Early emergence of cortical interneuron diversity in the mouse embryo. Science 360, 81–85 (2018).
Carter, R. A. et al. A single-cell transcriptional atlas of the developing murine cerebellum. Curr. Biol. 28, 2910–2920 (2018).
Guo, Q. & Li, J. Y. H. Defining developmental diversification of diencephalon neurons through single cell gene expression profiling. Development 146, dev174284 (2019).
Zhang, Y. et al. Cortical neural stem cell lineage progression is regulated by extrinsic signaling molecule Sonic hedgehog. Cell Rep. 30, 4490–4504 (2020).
Li, Z. et al. Transcriptional priming as a conserved mechanism of lineage diversification in the developing mouse and human neocortex. Sci. Adv. 6, eabd2068 (2020).
Ruan, X. et al. Progenitor cell diversity in the developing mouse neocortex. Proc. Natl Acad. Sci. USA 118, e2018866118 (2021).
Di Bella, D. J. et al. Molecular logic of cellular diversification in the mouse cerebral cortex. Nature 595, 554–559 (2021).
La Manno, G. et al. Molecular architecture of the developing mouse brain. Nature 596, 92–96 (2021).
Sarropoulos, I. et al. Developmental and evolutionary dynamics of cis-regulatory elements in mouse cerebellar cells. Science 373, eabg4696 (2021).
Turrero García, M. et al. Transcriptional profiling of sequentially generated septal neuron fates. eLife 10, e71545 (2021).
Farrell, J. A. et al. Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360, eaar3131 (2018).
Fienberg, H. G., Simonds, E. F., Fantl, W. J., Nolan, G. P. & Bodenmiller, B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry A 81A, 467–475 (2012).
Zunder, E. R. et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc. 10, 316–333 (2015).
Finck, R. et al. Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494 (2013).
Fread, K. I., Strickland, W. D., Nolan, G. P. & Zunder, E. R. An updated barcoding tool for mass cytometry with cell type-specific and cell-sample specific stringency adjustment. Pac. Symp. Biocomput. 22, 588–598 (2017).
Schuyler, R. P. et al. Minimizing batch effects in mass cytometry data. Front. Immunol. 10, 2367 (2019).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
McInnes, L., Healy, J., Saul, N. & Großberger, L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Schüller, U. et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14, 123–134 (2008).
Seto, Y., Ishiwata, S. & Hoshino, M. Characterization of Olig2 expression during cerebellar development. Gene Expr. Patterns 15, 1–7 (2014).
Riccio, O. et al. New pool of cortical interneuron precursors in the early postnatal dorsal white matter. Cereb. Cortex 22, 86–98 (2012).
Pfenninger, C. V. et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 67, 5727–5736 (2007).
Ono, K. et al. Regional- and temporal-dependent changes in the differentiation of Olig2 progenitors in the forebrain, and the impact on astrocyte development in the dorsal pallium. Dev. Biol. 320, 456–468 (2008).
Zheng, K. et al. Molecular and genetic evidence for the PDGFRα-independent population of oligodendrocyte progenitor cells in the developing mouse brain. J. Neurosci. 38, 9505–9513 (2018).
Kessaris, N. et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179 (2006).
VanRyzin, J. W. Phagocytic microglia in development: are they what they eat? Brain Behav. Immun. Health 18, 100373 (2021).
Qian, X. et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28, 69–80 (2000).
Gao, P. et al. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159, 775–788 (2014).
Verity, A. N. & Campagnoni, A. T. Regional expression of myelin protein genes in the developing mouse brain: in situ hybridization studies. J. Neurosci. Res. 21, 238–248 (1988).
Jakovcevski, I. & Zecevic, N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49, 480–491 (2005).
Englund, C. et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247–251 (2005).
Sessa, A. et al. The Tbr2 molecular network controls cortical neuronal differentiation through complementary genetic and epigenetic pathways. Cereb. Cortex 27, 3378–3396 (2017).
Zhou, Q., Wang, S. & Anderson, D. J. Identification of a novel family of oligodendrocyte lineage-specific basic helix–loop–helix transcription factors. Neuron 25, 331–343 (2000).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223 (2019).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Fu, Y. et al. Heterogeneity of glial progenitor cells during the neurogenesis-to-gliogenesis switch in the developing human cerebral cortex. Cell Rep. 34, 108788 (2021).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Angelo, M. et al. Multiplexed ion beam imaging (MIBI) of human breast tumors. Nat. Med. 20, 436–442 (2014).
Volovitz, I. et al. A non-aggressive, highly efficient, enzymatic method for dissociation of human brain-tumors and brain-tissues to viable single-cells. BMC Neurosci. 17, 30 (2016).
Guidelines for Mass Cytometry Panel Design White Paper No. PN 13-01_150711 (Fluidigm, 2015).
Acknowledgements
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS111220 to E.R.Z. and C.D.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Further support was provided by the 3Cavaliers Pilot research program to C.D.D. and E.R.Z. A.L.V.D. was supported by a UVA BRAIN Presidential Fellowship. S.K. was supported by the Owens Family Foundation. S.M.G. was supported by the National Institute of General Medical Sciences of the National Institutes of Health under the Systems and Biomedical Data Science Training Program, award number T32GM145443. C.M.W. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under the Cardiovascular Research Training Program, award number T32HL007284. A.B.K. was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number F32GM125147. K.I.F. was supported by the National Institute of General Medical Sciences of the National Institutes of Health under the Systems and Biomedical Data Science Training Program, award number T32GM136615. C.D.D. was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS091617. We thank A. Spano for contributing protein chemistry expertise and resources. We thank H. Zong (University of Virginia) for providing cell lines to validate antibodies and for providing feedback on the manuscript. We thank S. Kucenas (University of Virginia) for providing the anti-SOX10 antibody and feedback on the manuscript. We thank T. Müller and C. Birchmeier (Max Delbrück Center) for providing the anti-FABP7 antibody. We thank B. Condron, A. Güler, M. Hunter-Chang, A. Pathak, J. Sewell and E. Stepanova for providing feedback on the manuscript. We thank A. Hirt for assistance with coding and data analysis, K. Warner for assistance with antibody validation and L. Jin for assistance with mouse dissections. We thank the University of Virginia Flow Cytometry Core, RRID SCR_017829 for technical assistance with the CyTOF mass cytometer instrument. We acknowledge Research Computing at the University of Virginia (https://rc.virginia.edu) for providing computational resources and technical support that have contributed to the results reported within this publication.
Author information
Authors and Affiliations
Contributions
A.L.V.D., C.D.D. and E.R.Z. planned all experiments. A.L.V.D., A.B.K., I.C.G. and J.S. collected tissue and performed single-cell dissociations. A.L.V.D. performed all antibody conjugations. A.L.V.D. validated all antibodies by performing titration experiments on positive and negative control cell samples. A.L.V.D. and E.R.Z. performed the mass cytometry measurements. S.K. and O.Y.C. performed IHC and RNAscope ISH measurements. A.L.V.D., S.K. and J.S. performed microscopy. A.L.V.D., S.M.G., C.M.W, A.B.K., K.I.F. and E.R.Z. wrote scripts for data analysis. A.L.V.D., S.M.G. and E.R.Z. performed data analysis. E.R.Z. and C.D.D. conceived and supervised all aspects of the project. A.L.V.D., S.M.G. and E.R.Z. prepared figures. A.L.V.D., E.R.Z. and C.D.D. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Table 1
Antibodies used for mass cytometry.
Supplementary Fig. 1
Validation of single-cell processing of mouse brain tissue and titration of antibodies for mass cytometry.
Supplementary Fig. 2
Pre-processing of mass cytometry data for the developing mouse brain.
Supplementary Fig. 3
Classification of cells in the developing mouse brain by mass cytometry.
Supplementary Fig. 4
Comparison of protein expression in mouse brain measured by mass cytometry and immunohistochemistry.
Supplementary Fig. 5
Spatiotemporal profile of cell abundances in the developing mouse brain.
Supplementary Fig. 6
Comparison of protein and mRNA expression patterns in the developing mouse brain.
Supplementary Fig. 7
Differentiation trajectories of SOX2+Nestin+ cells in the developing mouse brain.
Supplementary Fig. 8
Differentiation trajectories and molecular dynamics in the telencephalon.
Supplementary Fig. 9
Microglia/macrophage expansion and putative phagocytic cargoes in the developing mouse brain.
Supplementary Data 1
Statistical source data for Supplementary Fig. 2.
Supplementary Data 2
Statistical source data for Supplementary Fig. 9.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Deusen, A.L., Kumar, S., Calhan, O.Y. et al. A single-cell mass cytometry-based atlas of the developing mouse brain. Nat Neurosci 28, 174–188 (2025). https://doi.org/10.1038/s41593-024-01786-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41593-024-01786-1