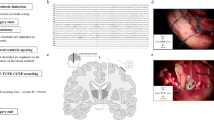
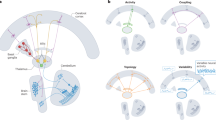
Abstract
The brain’s functional architecture is shaped by electrophysiological interactions between its components, encompassing both cortical and subcortical structures. In this study, we provide an atlas of electrophysiological causal connections across 4,864 brain sites in 27 human participants using repeated single-pulse electrical stimulations and recordings with intracranial electrodes implanted in cortical regions and multiple thalamic nuclei. We show distinct spectral signatures elicited by perturbations of specific brain areas. Identified features of causal connectivity exhibited highly organized yet distinct patterns, indicating that each feature may correspond to a separate mode of information transmission across brain regions. Notably, we report a new waveform with unique temporal and spatial characteristics specifically linked to thalamic stimulations, namely delayed-onset theta oscillations in both ipsilateral and contralateral cortical regions. These findings contribute to a more detailed understanding of the human brain’s functional architecture and offer valuable data for the development of biologically informed computational models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Preprocessed electrophysiological data for replicating the reported results are deposited at Zenodo and will be available upon publication (https://doi.org/10.5281/zenodo.15330862)62. The standard brain atlas used for group-level visualization is based on publicly available FS_LR brain surface: https://github.com/Washington-University/HCPpipelines/blob/master/global/templates/standard_mesh_atlases/. Source data are provided with this paper.
Code availability
All customized codes are deposited at Zenodo and will be available upon publication (https://doi.org/10.5281/zenodo.15330862)62. Any additional information about the current study can be requested from the corresponding author.
References
Sporns, O., Chialvo, D. R., Kaiser, M. & Hilgetag, C. C. Organization, development and function of complex brain networks. Trends Cogn. Sci. 8, 418–425 (2004).
Shine, J. M., Lewis, L. D., Garrett, D. D. & Hwang, K. The impact of the human thalamus on brain-wide information processing. Nat. Rev. Neurosci. 24, 416–430 (2023).
Matsumoto, R. et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain 127, 2316–2330 (2004).
Ojeda Valencia, G. et al. Signatures of electrical stimulation driven network interactions in the human limbic system. J. Neurosci. 43, 6697–6711 (2023).
Miller, K. J., Muller, K. R. & Hermes, D. Basis profile curve identification to understand electrical stimulation effects in human brain networks. PLoS Comput. Biol. 17, e1008710 (2021).
Jedynak, M. et al. Variability of single pulse electrical stimulation responses recorded with intracranial electroencephalography in epileptic patients. Brain Topogr. 36, 119–127 (2023).
Wu, T. Q. et al. Multisite thalamic recordings to characterize seizure propagation in the human brain. Brain 146, 2792–2802 (2023).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
Stieger, J. R. et al. Cross-regional coordination of activity in the human brain during autobiographical self-referential processing. Proc. Natl Acad. Sci. USA 121, e2316021121 (2024).
Call, C. L. & Bergles, D. E. Cortical neurons exhibit diverse myelination patterns that scale between mouse brain regions and regenerate after demyelination. Nat. Commun. 12, 4767 (2021).
Golden, E. C., Graff-Radford, J., Jones, D. T. & Benarroch, E. E. Mediodorsal nucleus and its multiple cognitive functions. Neurology 87, 2161–2168 (2016).
Togo, M. et al. Distinct connectivity patterns in human medial parietal cortices: evidence from standardized connectivity map using cortico-cortical evoked potential. Neuroimage 263, 119639 (2022).
Groppe, D. M. et al. Dominant frequencies of resting human brain activity as measured by the electrocorticogram. Neuroimage 79, 223–233 (2013).
Hacker, C. D., Snyder, A. Z., Pahwa, M., Corbetta, M. & Leuthardt, E. C. Frequency-specific electrophysiologic correlates of resting state fMRI networks. Neuroimage 149, 446–457 (2017).
Jones, E. G. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24, 595–601 (2001).
Groenewegen, H. J. & Berendse, H. W. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 17, 52–57 (1994).
Fisher, R. et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899–908 (2010).
Castro-alamancos, M. A. & Connors, B. W. Thalamocortical synapses. Prog. Neurobiol. 51, 581–606 (1997).
Gray, C. M. Synchronous oscillations in neuronal systems: mechanisms and functions. J. Comput. Neurosci. 1, 11–38 (1994).
Timofeev, I. & Chauvette, S. Thalamocortical oscillations: local control of EEG slow waves. Curr. Top. Med. Chem. 11, 2457–2471 (2011).
Jasper, H. Diffuse projection systems: the integrative action of the thalamic reticular system. Electroencephalogr. Clin. Neurophysiol. 1, 405–420 (1949).
Engel, J. Jr. & Pitkanen, A. Biomarkers for epileptogenesis and its treatment. Neuropharmacology 167, 107735 (2020).
Caciagli, L., Bernhardt, B. C., Hong, S.-J., Bernasconi, A. & Bernasconi, N. Functional network alterations and their structural substrate in drug-resistant epilepsy. Front. Neurosci. 8, 411 (2014).
Fleury, M. et al. Episodic memory network connectivity in temporal lobe epilepsy. Epilepsia 63, 2597–2622 (2022).
Pittau, F., Grova, C., Moeller, F., Dubeau, F. & Gotman, J. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 53, 1013–1023 (2012).
Roger, E. et al. Hubs disruption in mesial temporal lobe epilepsy. A resting-state fMRI study on a language-and-memory network. Hum. Brain Mapp. 41, 779–796 (2020).
Li, L. et al. Topographical reorganization of brain functional connectivity during an early period of epileptogenesis. Epilepsia 62, 1231–1243 (2021).
Mazrooyisebdani, M. et al. Graph theory analysis of functional connectivity combined with machine learning approaches demonstrates widespread network differences and predicts clinical variables in temporal lobe epilepsy. Brain Connect. 10, 39–50 (2020).
Ofer, I. et al. Association between seizure freedom and default mode network reorganization in patients with unilateral temporal lobe epilepsy. Epilepsy Behav. 90, 238–246 (2019).
Liao, W. et al. Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Hum. Brain Mapp. 32, 883–895 (2011).
Widjaja, E., Zamyadi, M., Raybaud, C., Snead, O. C. & Smith, M. L. Abnormal functional network connectivity among resting-state networks in children with frontal lobe epilepsy. AJNR Am. J. Neuroradiol. 34, 2386–2392 (2013).
Zhang, Z. et al. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci. Lett. 458, 97–101 (2009).
McGinn, R. et al. Ictal involvement of the pulvinar and the anterior nucleus of the thalamus in patients with refractory epilepsy. Neurology 103, e210039 (2024).
Buzsaki, G. Rhythms of the Brain (Oxford University Press, 2006).
Penttonen, M. & Buzsáki, G. Natural logarithmic relationship between brain oscillators. Thalamus Relat. Syst. 2, 145–152 (2003).
Steriade, M. Impact of network activities on neuronal properties in corticothalamic systems. J. Neurophysiol. 86, 1–39 (2001).
Miller, K. J., Sorensen, L. B., Ojemann, J. G. & Den Nijs, M. Power-law scaling in the brain surface electric potential. PLoS Comput. Biol. 5, e1000609 (2009).
Buzsaki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Keller, C. J. et al. Mapping human brain networks with cortico-cortical evoked potentials. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130528 (2014).
Veit, M. J. et al. Temporal order of signal propagation within and across intrinsic brain networks. Proc. Natl Acad. Sci. USA 118, e2105031118 (2021).
Seguin, C. et al. Communication dynamics in the human connectome shape the cortex-wide propagation of direct electrical stimulation. Neuron 111, 1391–1401 (2023).
Guo, Z. H. et al. Epileptogenic network of focal epilepsies mapped with cortico-cortical evoked potentials. Clin. Neurophysiol. 131, 2657–2666 (2020).
Kunieda, T., Yamao, Y., Kikuchi, T. & Matsumoto, R. New approach for exploring cerebral functional connectivity: review of cortico-cortical evoked potential. Neurol. Med. Chir. (Tokyo) 55, 374–382 (2015).
Keller, C. J. et al. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc. Natl Acad. Sci. USA 108, 10308–10313 (2011).
Arthuis, M. et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain 132, 2091–2101 (2009).
Guye, M. et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain 129, 1917–1928 (2006).
Pizzo, F. et al. The ictal signature of thalamus and basal ganglia in focal epilepsy: a SEEG study. Neurology 96, e280–e293 (2021).
Filipescu, C. et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia 60, e25–e30 (2019).
Evangelista, E. et al. Does the thalamo-cortical synchrony play a role in seizure termination? Front Neurol. 6, 192 (2015).
Gadot, R., Korst, G., Shofty, B., Gavvala, J. R. & Sheth, S. A. Thalamic stereoelectroencephalography in epilepsy surgery: a scoping literature review. J. Neurosurg. 137, 1210–1225 (2022).
Ilyas, A., Tandon, N. & Lhatoo, S. D. Thalamic neuromodulation for epilepsy: a clinical perspective. Epilepsy Res. 183, 106942 (2022).
Chaitanya, G. et al. Robot-assisted stereoelectroencephalography exploration of the limbic thalamus in human focal epilepsy: implantation technique and complications in the first 24 patients. Neurosurg. Focus 48, E2 (2020).
Romeo, A. et al. Early ictal recruitment of midline thalamus in mesial temporal lobe epilepsy. Ann. Clin. Transl. Neurol. 6, 1552–1558 (2019).
McKhann, G. M. Editorial. Dulling the double-edged sword of human SEEG research. Neurosurg. Focus 48, E3 (2020).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. Fsl. Neuroimage 62, 782–790 (2012).
Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001).
Papademetris, X. et al. BioImage suite: an integrated medical image analysis suite: an update. Insight J. 2006, 209 (2006).
Groppe, D. M. et al. iELVis: an open source MATLAB toolbox for localizing and visualizing human intracranial electrode data. J. Neurosci. Methods 281, 40–48 (2017).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at arXiv https://doi.org/10.48550/arXiv.1802.03426 (2020).
McInnes, L. et al. hdbscan: Hierarchical density based clustering. J. Open Source Softw. 2, 205 (2017).
Lyu, D. Causal cortical and thalamic connections in the human brain. Zenodo https://doi.org/10.5281/zenodo.15330862 (2025).
Acknowledgements
We are grateful to the patients who agreed to participate in our research, as well as to the staff in the EEG lab, including clinical fellows and attending physicians, whose assistance enabled this research. We express our gratitude to our colleagues R. Matsumoto (University of Kyoto, Kyoto, Japan), C. Keller (Stanford University, Palo Alto, CA, USA), K. Körding (University of Pennsylvania, Philadelphia, PA, USA) and R. T. Knight (University of California, Berkeley, Berkeley, CA, USA) for their valuable feedback; and to L. McInnes (Ottawa, Canada) for running a sanity check on our UMAP analysis approach. This work was supported by research grants from the US National Institute of Neurological Disorders and Stroke (R01NS078396 and R21NS113024), US National Institute of Mental Health (1R01MH109954 and P50MH109429) and US National Science Foundation (BCS1358907 and BCS1850938 to J.P.).
Author information
Authors and Affiliations
Contributions
D.L. contributed to the conception, acquisition, analysis and interpretation of data, and creation of new codes and measures used in the work and writing of the manuscript. J.R.S. contributed to the analysis and interpretation of data. Z.L. contributed to the acquisition of data. V.B. performed surgeries and contributed to the acquisition and interpretation of data and the editing of the manuscript. J.P. contributed to the conception and design of the work, the acquisition and interpretation of data and the writing of the manuscript. All authors provided significant feedback throughout the study and manuscript preparation and have approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Riki Matsumoto, Nicholas Schiff and other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Supplementary Tables 1–9.
Supplementary Table 8
Significance testing results for all post hoc comparisons with either feature presentation (‘Fx_peak’) or feature latency (‘Fx_time’) as dependent variables (x being 1, 2 and 3).
Source data
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyu, D., Stiger, J.R., Lusk, Z. et al. Mapping human thalamocortical connectivity with electrical stimulation and recording. Nat Neurosci 28, 1797–1809 (2025). https://doi.org/10.1038/s41593-025-02009-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41593-025-02009-x
This article is cited by
-
Direct interactions between the human insula and hippocampus during memory encoding
Nature Neuroscience (2025)