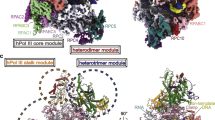
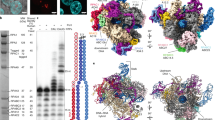
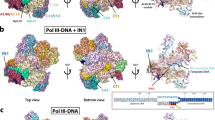
Abstract
RNA polymerase III (Pol III) synthesizes transfer RNAs and other short, essential RNAs. Human Pol III misregulation is linked to tumor transformation, neurodegenerative and developmental disorders, and increased sensitivity to viral infections. Here, we present cryo-electron microscopy structures at 2.8 to 3.3 Å resolution of transcribing and unbound human Pol III. We observe insertion of the TFIIS-like subunit RPC10 into the polymerase funnel, providing insights into how RPC10 triggers transcription termination. Our structures resolve elements absent from Saccharomyces cerevisiae Pol III such as the winged-helix domains of RPC5 and an iron–sulfur cluster, which tethers the heterotrimer subcomplex to the core. The cancer-associated RPC7α isoform binds the polymerase clamp, potentially interfering with Pol III inhibition by tumor suppressor MAF1, which may explain why overexpressed RPC7α enhances tumor transformation. Finally, the human Pol III structure allows mapping of disease-related mutations and may contribute to the development of inhibitors that selectively target Pol III for therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Cryo-EM maps of human Pol III (map A to map G) have been deposited at the Electron Microscopy Data Bank (EMDB) under accession codes EMD-11673 (map A) and EMD-11736 to EMD-11742 (map B to H). Atomic models of human Pol III have been deposited at the Protein Data Bank under accession codes 7A6H (apo Pol III), 7AE1 (EC-1 Pol III), 7AEA (EC-2 Pol III) and 7AE3 (EC-3 Pol III). Source data are provided with this paper.
References
Arimbasseri, A. G. & Maraia, R. J. RNA polymerase III advances: structural and tRNA functional views. Trends Biochem. Sci. 41, 546–559 (2016).
Willis, I. M. & Moir, R. D. Signaling to and from the RNA polymerase III transcription and processing machinery. Annu. Rev. Biochem. 87, 75–100 (2018).
Lei, J., Chen, S. & Zhong, S. Abnormal expression of TFIIIB subunits and RNA Pol III genes is associated with hepatocellular carcinoma. Liver Res. 1, 112–120 (2017).
Petrie, J. L. et al. Effects on prostate cancer cells of targeting RNA polymerase III. Nucleic Acids Res. 47, 3937–3956 (2019).
Filer, D. et al. RNA polymerase III limits longevity downstream of TORC1. Nature 552, 263–267 (2017).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072 (2009).
Chiu, Y.-H., MacMillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Bernard, G. et al. Mutations of POLR3A encoding a catalytic subunit of RNA polymerase Pol III cause a recessive hypomyelinating leukodystrophy. Am. J. Hum. Genet. 89, 415–423 (2011).
Tétreault, M. et al. Recessive mutations in POLR3B, encoding the second largest subunit of Pol III, cause a rare hypomyelinating leukodystrophy. Am. J. Hum. Genet. 89, 652–655 (2011).
Thiffault, I. et al. Recessive mutations in POLR1C cause a leukodystrophy by impairing biogenesis of RNA polymerase III. Nat. Commun. 6, 7623 (2015).
Saitsu, H. et al. Mutations in POLR3A and POLR3B encoding RNA polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy. Am. J. Hum. Genet. 89, 644–651 (2011).
Gauquelin, L. et al. Clinical spectrum of POLR3-related leukodystrophy caused by biallelic POLR1C pathogenic variants. Neurol. Genet. 5, e369 (2019).
Wolf, N. I. et al. Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology 83, 1898–1905 (2014).
Dorboz, I. et al. Mutation in POLR3K causes hypomyelinating leukodystrophy and abnormal ribosomal RNA regulation. Neurol. Genet. 4, e289 (2018).
Paolacci, S. et al. Specific combinations of biallelic POLR3A variants cause Wiedemann–Rautenstrauch syndrome. J. Med. Genet. 55, 837–846 (2018).
Wambach, J. A. et al. Bi-allelic POLR3A loss-of-function variants cause autosomal-recessive Wiedemann–Rautenstrauch syndrome. Am. J. Hum. Genet. 103, 968–975 (2018).
Dauwerse, J. G. et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat. Genet. 43, 20–22 (2011).
Ogunjimi, B. et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J. Clin. Invest. 127, 3543–3556 (2017).
Carter-Timofte, M. E. et al. Mutations in RNA Polymerase III genes and defective DNA sensing in adults with varicella-zoster virus CNS infection. Genes Immun. 127, 3543–3556 (2017).
Vannini, A. & Cramer, P. Conservation between the RNA polymerase I, II, and III transcription initiation machineries. Mol. Cell 45, 439–446 (2012).
Brun, I., Sentenac, A. & Werner, M. Dual role of the C34 subunit of RNA polymerase III in transcription initiation. EMBO J. 16, 5730–5741 (1997).
Wang, Z. & Roeder, R. G. Three human RNA polymerase III-specific subunits form a subcomplex with a selective function in specific transcription initiation. Genes Dev. 11, 1315–1326 (1997).
Lefèvre, S. et al. Structure-function analysis of hRPC62 provides insights into RNA polymerase III transcription initiation. Nat. Struct. Mol. Biol. 18, 352–358 (2011).
Hu, P. et al. Characterization of human RNA polymerase III identifies orthologues for Saccharomyces cerevisiae RNA polymerase III subunits. Mol. Cell. Biol. 22, 8044–8055 (2002).
Kassavetis, G. A., Prakash, P. & Shim, E. The C53/C37 subcomplex of RNA polymerase III lies near the active site and participates in promoter opening. J. Biol. Chem. 285, 2695–2706 (2010).
Arimbasseri, A. G. & Maraia, R. J. Mechanism of transcription termination by RNA polymerase III utilizes a non-template strand sequence-specific signal element. Mol. Cell 58, 1124–1132 (2015).
Landrieux, E. et al. A subcomplex of RNA polymerase III subunits involved in transcription termination and reinitiation. EMBO J. 25, 118–128 (2006).
Chédin, S., Riva, M., Schultz, P., Sentenac, A. & Caries, C. The RNA cleavage activity of RNA polymerase III is mediated by an essential TFIIS-like subunit and is important for transcription termination. Genes Dev. 12, 3857–3871 (1998).
Iben, J. R. et al. Point mutations in the Rpb9-homologous domain of Rpc11 that impair transcription termination by RNA polymerase III. Nucleic Acids Res. 39, 6100–6113 (2011).
Blombach, F. et al. Archaeal TFEα/β is a hybrid of TFIIE and the RNA polymerase III subcomplex hRPC62/39. Elife 4, e08378 (2015).
Haurie, V. et al. Two isoforms of human RNA polymerase III with specific functions in cell growth and transformation. Proc. Natl Acad. Sci. USA 107, 4176–4181 (2010).
Hoffmann, N. A. et al. Molecular structures of unbound and transcribing RNA polymerase III. Nature 528, 231–236 (2015).
Abascal-Palacios, G., Ramsay, E. P., Beuron, F., Morris, E. & Vannini, A. Structural basis of RNA polymerase III transcription initiation. Nature 553, 301–306 (2018).
Vorländer, M. K., Khatter, H., Wetzel, R., Hagen, W. J. H. & Müller, C. W. Molecular mechanism of promoter opening by RNA polymerase III. Nature 553, 295–300 (2018).
Han, Y., Yan, C., Fishbain, S., Ivanov, I. & He, Y. Structural visualization of RNA polymerase III transcription machineries. Cell Discov. 4, 40 (2018).
Vorländer, M. K. et al. Structural basis for RNA polymerase III transcription repression by Maf1. Nat. Struct. Mol. Biol. 27, 229–232 (2020).
Bernecky, C., Herzog, F., Baumeister, W., Plitzko, J. M. & Cramer, P. Structure of transcribing mammalian RNA polymerase II. Nature 529, 551–554 (2016).
Kettenberger, H., Armache, K. J. & Cramer, P. Complete RNA polymerase II elongation complex structure and its interactions with NTP and TFIIS. Mol. Cell 16, 955–965 (2004).
Wang, D. et al. Structural basis of transcription: backtracked RNA polymerase II at 3.4 Angstrom resolution. Science 324, 1203–1206 (2009).
Cheung, A. C. M. & Cramer, P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature 471, 249–253 (2011).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. Preprint at bioRxiv https://doi.org/10.1101/2020.04.08.032466 (2020).
Huang, Y., Intine, R. V., Mozlin, A., Hasson, S. & Maraia, R. J. Mutations in the RNA Polymerase III subunit Rpc11p that decrease RNA 3′ cleavage activity increase 3′-terminal oligo(U) length and La-dependent tRNA processing. Mol. Cell. Biol. 25, 621–636 (2005).
Mishra, S. & Maraia, R. J. RNA polymerase III subunits C37/53 modulate rU:dA hybrid 3′ end dynamics during transcription termination. Nucleic Acids Res. 47, 310–327 (2019).
Dieci, G. & Sentenac, A. Facilitated recycling pathway for RNA polymerase III. Cell 84, 245–252 (1996).
He, Y. et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533, 359–365 (2016).
Wang, Z., Wu, C., Aslanian, A., Yates, J. R. & Hunter, T. Defective RNA polymerase III is negatively regulated by the SUMO-Ubiquitin-Cdc48 pathway. Elife 7, e35447 (2018).
Sadian, Y. et al. Molecular insight into RNA polymerase I promoter recognition and promoter melting. Nat. Commun. 10, 5543 (2019).
Dergai, O. & Hernandez, N. How to recruit the correct RNA polymerase? Lessons from snRNA genes. Trends Genet. 35, 457–469 (2019).
Renaud, M. et al. Gene duplication and neofunctionalization: POLR3G and POLR3GL. Genome Res. 24, 37–51 (2014).
Durrieu-Gaillard, S. et al. Regulation of RNA polymerase III transcription during transformation of human IMR90 fibroblasts with defined genetic elements. Cell Cycle 17, 605–615 (2018).
Davis, R. B., Likhite, N., Jackson, C. A., Liu, T. & Yu, M. C. Robust repression of tRNA gene transcription during stress requires protein arginine methylation. Life Sci. Alliance 2, e201800261 (2019).
Maraia, R. J. & Rijal, K. A transcriptional specialist resolved. Nature 528, 204–205 (2015).
Koch, B. et al. Generation and validation of homozygous fluorescent knock-in cells using CRISPR–Cas9 genome editing. Nat. Protoc. 13, 1465–1487 (2018).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Lin, Y. C. et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 5, 4767 (2014).
Kim, J. H. et al. High cleavage efficiency of a 2A peptide derived from porcine Teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 6, e18556 (2011).
Moreno-Morcillo, M. et al. Solving the RNA polymerase I structural puzzle. Acta Crystallogr. D Biol. Crystallogr. 70, 2570–2582 (2014).
Federhen, S. The NCBI Taxonomy Database. Nucleic Acids Res. 40, D136–D143 (2012).
The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
El-Gebali, S. et al. The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427–D432 (2019).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Kayikci, M. et al. Visualization and analysis of non-covalent contacts using the Protein Contacts Atlas. Nat. Struct. Mol. Biol. 25, 185–194 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Csárdi, G. & Nepusz, T. The igraph software package for complex network research. Inter. J. Comp. Syst. 1695, 1–9 (2006).
Mitternacht, S. FreeSASA: an open source C library for solvent accessible surface area calculations. F1000 Res. 5, 189 (2016).
Nakane, T., Kimanius, D., Lindahl, E. & Scheres, S. H. W. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. Elife 7, e36861 (2018).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Hwang, S., Gou, Z. & Kuznetsov, I. B. DP-Bind: a web server for sequence-based prediction of DNA-binding residues in DNA-binding proteins. Bioinformatics 23, 634–636 (2007).
Hornbeck, P. V. et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520 (2015).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Kim, M. K. et al. Assembly of SNAPc, Bdp1, and TBP on the U6 snRNA gene promoter in Drosophila melanogaster. Mol. Cell. Biol. 40, e00641-19 (2020).
Notredame, C., Higgins, D. G. & Heringa, J. T-coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 (2000).
Madeira, F. et al. The EMBL–EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, W636–W641 (2019).
Acknowledgements
We thank F. Dossin for giving valuable advice on CRISPR–Cas9 and sharing a plasmid used in gene editing, F. Weis and W. J. H. Hagen (EMBL Cryo-Electron Microscopy Service Platform) for EM support, P. Haberkant and M. Rettel (EMBL Proteomics Core Facility) for carrying out MS analysis, T. Hoffmann and J. Pecar for setting up and maintaining the high-performance computing environment, and current members of the Müller laboratory and S. Eustermann for discussions. M.G. was supported by a Boehringer Ingelheim Fonds PhD fellowship. M.G., A.D.M. and A.L. acknowledge support from the EMBL International PhD program.
Author information
Authors and Affiliations
Contributions
C.W.M. initiated and supervised the project. M.G. initiated the project; carried out EM grid preparation, data collection and processing and model building; and interpreted the structures. A.D.M. generated HEK293F cell lines via CRISPR–Cas9-mediated gene editing and purified proteins and analyzed Pol III mutations. M.K.V. assisted with structural model building, interpretation of the structures and advice for EM data processing. A.L. and A.B. performed the phylogenomic analysis. F.B. did the RNA extension and cleavage assays. H.G. performed cell culture work and advised and assisted with CRISPR–Cas9-mediated gene editing and protein purification. M.G., A.D.M. and C.W.M. wrote the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature Structural & Molecular Biology thanks the reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Extended data
Extended Data Fig. 1 Endogenous tagging of RPAC1 and analysis of purified Pol III.
a, Zygosity PCR with purified genomic DNA from WT cells HEK293T and produced RPAC1-Strep II-mCherry tagged cell line. The experiment was performed twice. b, Western blot of cell lysates from WT cells and positive for the insert cell line probed with anti-mCherry antibody (Abcam). The experiment was performed once. c, Representative negative stain micrograph showing homogenous sample. d, Coomassie-stained SDS-PAGE of purified Pol III complex. Identities of the labelled bands were confirmed by mass spectrometry with the highest scoring hit shown. For RPC7 (starred) the α isoform was detected as most abundant, while β isoform was also present in the sample. e, Primer extension assay confirming the activity of the purified complex. 5’ radioactively tagged RNA primer (starred) assembled with the DNA scaffold (cartoon representation) was used. Arrows indicate the extended primer products. For experimental details, see Methods. Uncropped images for all panels are shown in the Source Data.
Extended Data Fig. 2 Global Cryo-EM data processing strategy of apo and elongating human Pol III.
a, Talos Arctica screening dataset, from which a low-resolution 3D reconstruction of apo human Pol III at 8 Å was obtained. b, Processing pipeline of the Titan Krios data set of apo human Pol III. For global 3D classification, the low-resolution Apo Pol III map (filtered to 60 Å) was used as a reference model. c, Processing pipeline of the Titan Krios data set of elongating human Pol III. For global 3D classification, the high-resolution Apo Pol III map (filtered to 60 Å) was used as a reference model. High-resolution cryo-EM maps were further subjected to RELION multi-body refinement67 with applied masks (transparent surfaces) covering the core and heterodimer (green maps A1, B1) and the clamp, heterotrimer and stalk domains (purple maps A2, B2). Representative micrographs of the three datasets are shown on the left. Shown are the unsharpened cryo-EM maps whereas reported resolution values correspond to automatically B-factor sharpened maps obtained via RELION post-processing. Resolution values of the unsharpened maps are added with parenthesis. Shown particle numbers were rounded down. All maps, marked with colored boxes, were used for structural model building.
Extended Data Fig. 3 Refined cryo-EM data processing strategy to improve signals of flexible regions.
Conformation heterogeneity was resolved using masked classification in RELION. Coloured unfilled circles mark regions with applied masks. Filled, transparent circles highlight elements with improved cryo-EM density. Threshold levels of the shown maps were individually adjusted to visualise flexible elements. Maps labelled with coloured boxes were used for structural model building. Map G and H derived from a merged dataset of apo and elongating human Pol III. Both RELION68 and CryoSPARC69 were used for data processing, as indicated.
Extended Data Fig. 4 Cryo-EM data quality assessment.
a, FSC curves of the apo and elongating Pol III showing final average resolutions of 3.3Å and 2.8Å, respectively (FSC = 0.143). b, FSC curves of cryo-EM maps with resolved flexible regions seen in Extended Data Fig. 3. Nominal resolution values are given in brackets (FSC = 0.143). Local resolution estimation of c, apo Pol III and d, elongating Pol III as implemented in RELION. Corresponding angular distribution plots of all particles contributing to apo and elongating Pol III structures are given on the right-hand side of c, and d, panels respectively. Fit of the modelled representative active site elements (stick representation): e, wall, f, bridge helix and g, trigger loop into its corresponding cryo-EM densities (surface representation). h, Fit of the nucleic acids into its corresponding cryo-EM density (coloured surface representation). Low-pass filtered by Gaussian filter = 1.5 density shown in mesh representation.
Extended Data Fig. 5 Cryo-EM density around newly built regions of human RPC4, RPC5 and RPC7.
a, Newly built RPC4 dimerization domain shown in molecular context (cartoon). b, RPC5 C-terminal domain, built into map F, is anchored to RPC2 and RPC4. c, Stalk bridge connecting the heterotrimer. Black mesh representation shows a 5 Å low-pass filtered cryo-EM density. Key interacting residues Y87 of RPC7 and R107 of RPC9 are shown in a close-up. The corresponding cryo-EM density is shown as blue mesh and belongs to map B2 (RELION multi-body refinement on elongating Pol III) sharpened by density modification.
Extended Data Fig. 6 Structures of human RPC10 in different conformations.
Shown are cartoon representations of human RPC10 or its homologs in yeast, of nucleic acids and of the polymerase regions that form the pore and funnel domains and the jaw domain. Acidic hairpins of RPC10, C11 and TFIIS are coloured dark red. a, RPC10 C-ribbon (C) adopting the ‘outside funnel’ conformation in the elongating Pol III structure. b, Cryo-EM density (map E) is depicted as transparent grey surface and shows the fit of the C-ribbon of RPC10 in the ‘outside funnel’ conformation. c, C11 (the yeast homolog to RPC10) shows a different conformation of its C-ribbon domain, whereas the position of the N-ribbon (N) is similar in both species. Shown is the yeast apo Pol III structure (PDB 5fja) because the C-ribbon is not visible other conformational states. d, The RPC10 C-ribbon in the ‘outside funnel’ conformation folds back and bind its linker and the RPC1 jaw. RPC10 from residues 83 to 91 are shown as ribbon, and interacting residues within 4 Å distance are shown as sticks (left). Sequence alignments of H. sapiens RPC1 and S. cerevisiae C160 and of H. sapiens RPC10 and S. cerevisiae C11 covering the respective regions (right) shows that the contacting residues are not conserved. e, RNA cleavage assay of the 32P-labelled RNA annealed to the same transcription scaffold as used for structure determination of elongating Pol III. Shown is a time course from 0 to 120 minutes. Lane 0* contains RNA in the absence of polymerase. Hs – H. sapiens; Sc – S. cerevisiae. Uncropped image is shown in the Source Data. f, RPC10 (C) adopting the ‘inside funnel’ conformation (EC-3 Pol III) upon straightening of the linker. g, Cryo-EM density (map D) is depicted as transparent grey surface and shows the fit of the C-ribbon of RPC10 in the ‘inside funnel’ conformation. h, Structure of yeast TFIIS bound to Pol II in its ‘reactivation intermediate’ conformation (PDB 3PO3). i, j, Comparison between the structures of i, human Pol III RPC10 in the ‘inside funnel’ conformation and j, yeast Pol II bound to TFIIS (PDB 3PO3). Close-up views of RPC10 and TFIIS reaching into the active are shown on the right. RPC10 doesn’t reach as far into the polymerase active site as TFIIS. The acidic hairpin is inflected in RPC10 and is extended in TFIIS. Gating tyrosines of human Pol III RPC2 (Y684) and yeast Pol II RPB2 (Y769) are shown as sticks and adopt different conformations.
Extended Data Fig. 7 Structural and computational analysis of RPC4-RPC5 extensions.
a, Schematic domain organization of human RPC4 and RPC5 with DNA binding predictions below as predicted by DP-Bind70. Sumo-modifications of human RPC4 (K78, K141, K190, K199, K206, K220, K285, K288, K302) are marked on the sequence and were obtained from the PhosphoSitePlus database71. Dashed lines indicate regions that were not visible in the human Pol III cryo-EM maps. Human RPC5 contains, according to sequence homology prediction72, four WH domains that are absent in S. cerevisiae. b, Close-up view onto K141 of RPC4 being buried within the interface of RPC4 DM* (shown as cartoon), RPC5 and RPC10 (shown as transparent surfaces). c, Structural model of newly built RPC5 WH1 and WH2 domains binding the Pol III core subunits RPC2, RPAC1, and RPABC5. Corresponding contact points are labelled. d, Conformational dynamics of the WH1-WH2 domains. Left: Cryo-EM maps of human Pol III, in which RPC5 WH domains adopt conformation 1 (map F, top) and conformation 2 (map H, bottom). Cryo-EM maps were low-pass filtered to 6 Å and are shown as transparent surfaces. RPC4 and RPC5 are shown as thick ribbons. Right: Superimposition of the WH domains in the two conformations. The black arrow illustrates their movement. The WH domains in conformation 2 are shown as transparent cartoons e, Phylogenetic analysis of RPC5 and its WH domains, of TFIIIB components Brf1 and Brf2, as well as of the SNAPc genes. The color code for RPC5 is the same as in a. Coloured and white squares indicate identified and not-identified homologues, respectively. The stars mark species, in which all the SNAPc building blocks were found that are likely required to form a functional SNAPc complex (SNAPc1, SNAPc3, SNAPc4) For further details of phylogenetic analysis see Methods. f, Model of the human Pol III PIC assembled onto the U6 snDNA promoter. Pol III core is shown as surface and RPC5 WH domains, Brf2, Bdp1, TBP and DNA are represented as cartoons. SNAPc is illustrated as transparent sphere. The model was generated using Chimera and Coot by: i) superimposing human TBP from PDB 5n9g onto yeast TBP in PDB 6f42, ii) extending the upstream DNA in 5n9g manually with ideal B-DNA carrying the human U6 snDNA sequence in Coot, iii) superimposing the elongating human Pol III model onto the model of yeast Pol III with Chain C (RPAC1) being used for alignment. SNAPc was placed onto the modelled downstream DNA according to information retrieved from the literature48,73.
Extended Data Fig. 8 Structure, function and evolution the RPC6 FeS domain and RPC3 extensions.
a, Multiple sequence alignment (MSA) of the RPC6 C-terminal FeS domain from model organisms. MSA was generated via T-Coffee74 implemented into the EMBL-EBI bioinformatic tools web service75. The four cysteine residues required for coordination of the FeS cluster are highlighted in red. b, Comparison of the RPC3-RPC6-RPC7 heterotrimer from H. sapiens (left, elongating Pol III) and S. cerevisiae (right, PDB 6eu3). The cubane FeS cluster is coloured in red and yellow. The position of the FeS cluster is framed, and a close-up view shows the four cysteines coordinating the FeS cluster. The equivalent position in S. cerevisiae Pol III structure, which lacks the FeS cluster, is marked with a dashed oval. c, MSA of the RPC3 (C82 in S. cerevisiae) N-terminus and of region 275–294 (H. sapiens) generated with T-Coffee. The MSA of the RPC3 N-terminus was manually adjusted, based on the available structural information (see panel d), so that the N-terminus of budding yeasts becomes apparent as an N-terminal extension instead of an insertion. The budding yeast specific N-terminal extension and central insertion (331–354 in S. cerevisiae) are highlighted with pink frames. d-f, Close-up comparisons between H. sapiens (elongating Pol III) and S. cerevisiae (PDB 6eu3 in d, e and 6f41 in f) Pol III structural elements that contribute to heterotrimer integrity and anchoring to the RPC1 (C160 in S. cerevisiae) core subunit. d, Budding yeast-specific N-terminal extension is positioned similarly as the FeS cluster in H. sapiens Pol III. Notably, only 16 residues (31-46) of the extension are visible, whereas the remaining amino acids are flexible. e, The central insertion (331-354) binds the polymerase core, whereas this contact point is missing in H. sapiens. f, S. cerevisiae Pol III contains a helix-turn-helix (HTH) insertion (202-258) that binds the downstream DNA in the S. cerevisiae PIC (PDB 6f41). The HTH insertion further binds and stabilises the central insertion (331-354) and, thereby, likely contributes to the anchoring of the heterotrimer to the polymerase core. Both elements are absent in H. sapiens although the counterpart region (159-187 in H. sapiens) has a similar shape (albeit without α-helical elements) that points into a different direction. Hs – H. sapiens; Sc – S. cerevisiae. Sc RPC1, Sc RPC3, Sc RPC6 relate to Sc C160, Sc C82, Sc C34, respectively, according to the commonly used nomenclature of S. cerevisiae Pol III subunits.
Extended Data Fig. 9 RPC7 tethers heterotrimer and stalk that move in opposite directions.
Protein elements are shown as thick ribbon to better visualize conformational changes. a, Left: Schematic showing the transition from DNA-unbound Apo Pol III to DNA/RNA-bound elongating Pol III. Middle: Side view showing the superimposition of apo and elongating human Pol III with RPAC1 being used for aligning the two structures. Apo Pol and elongating human Pol III are shown as transparent and solid ribbons, respectively. Green arrows illustrate movements of Pol III heterotrimer and clamp and the polymerase stalk domain. Right: Close-up views on selected moving elements showing that heterotrimer and clamp move towards the bound downstream DNA upon transition from apo to elongating Pol III. b, c, Top views onto the heterotrimer and stalk domains with two different subunits (RPAC1 in b and RPC3 in c) used to align apo and elongating human Pol III structures. Aligned subunits are outlined in bold in the associated schematics shown on the left. b, The RPC7 stalk bridge tethers heterotrimer and stalk although they the two domains move in opposite directions. c, The tip of the RPC7 stalk bridge is anchored to the stalk domain and moves in the same direction.
Extended Data Fig. 10 Outlined types of point mutations in Pol III correlate with diseases.
a, Cartoon representation of Pol III showing affected subunits (in colour). Diseases correlated with mutations and types they have been assigned to. b, Relative distribution of types of mutations in each considered disease. c, Relative distribution of types of mutations in each subunit. Coloured outlines reference diseases. Size of bars corresponds to the number of mutations within each subunit. For the type assignment, see Methods. For details of individual mutations, see Supplementary Table 2.
Supplementary information
Supplementary Information
Supplementary Note 1, Tables 1–4 and References.
Supplementary Video 1
Video of 3D variability analysis.
Source data
Source Data Extended Data Fig. 1
Unprocessed source data.
Source Data Extended Data Fig. 6
Unprocessed source data.
Rights and permissions
About this article
Cite this article
Girbig, M., Misiaszek, A.D., Vorländer, M.K. et al. Cryo-EM structures of human RNA polymerase III in its unbound and transcribing states. Nat Struct Mol Biol 28, 210–219 (2021). https://doi.org/10.1038/s41594-020-00555-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-020-00555-5
This article is cited by
-
Challenges and opportunities in technologies and methods for lncRNA structure determination
Cell & Bioscience (2025)
-
Iron-sulfur clusters in SARS-CoV-2 exoribonuclease and methyltransferase complexes: relevance for viral genome proofreading and capping
Nature Communications (2025)
-
Structural insights into distinct mechanisms of RNA polymerase II and III recruitment to snRNA promoters
Nature Communications (2025)
-
A large-scale curated and filterable dataset for cryo-EM foundation model pre-training
Scientific Data (2025)
-
Structural insights into human Pol III transcription initiation in action
Nature (2025)