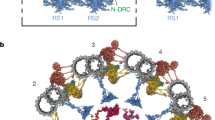
Abstract
Cilia are microtubule-based organelles that have important roles in cell sensing, signaling and motility. Recent studies have revealed the atomic structures of many multicomponent ciliary complexes, elucidating their mechanisms of action. However, little is known about the structure, proteome and function of full-length radial spoke 3 (RS3), a conserved complex that transmits mechanochemical signals to coordinate ciliary motility. Here, we combined single-particle cryo-electron microscopy, cryo-electron tomography, proteomic analysis and computational modeling to determine the three-dimensional structure and atomic model of RS3 from mouse respiratory cilia. We reveal all RS3 components, including regulatory and metabolic enzymes such as a protein kinase A subunit, adenylate kinases (AKs) and malate dehydrogenases. Furthermore, we confirm RS3 loss in AK7-deficient mice, which exhibit motility defects. Our findings identify RS3 as an important regulatory and metabolic hub that maintains sufficient adenosine triphosphate for sustained ciliary beating, providing insights into the etiology of ciliopathies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The UniProt database and AlphaFold protein structure database were used for proteomic analysis and modeling, respectively. All cryo-EM maps and models reported in this work were deposited to the EM Data Bank under accession codes EMD-46484 (WT), EMD-46486 (AK7−/−), EMD-46485 (AK1−/−) and EMD-46494 (PDB 9D2F; composite cryo-EM density map and atomic coordinate of RS3 from mouse respiratory cilia). The original proteomics data for the Tetrahymena cilia and the mouse respiratory cilia were deposited to the approved data repository ProteomeXchange Consortium through the MassIVE partner repository with dataset identifiers MSV000096999 and MSV000096704, respectively. Source data are provided with this paper.
Code availability
The Python scripts CryoAlphaID for running the COLORES-based search, EMAN2-based FSC resolution calculation and ranking of the models are available from GitHub (https://github.com/xuewuzhang-UTSW/CryoAlphaID).
References
Afzelius, B. A. A human syndrome caused by immotile cilia. Science 193, 317–319 (1976).
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893 (2007).
Brown, J. M. & Witman, G. B. Cilia and diseases. Bioscience 64, 1126–1137 (2014).
Pazour, G. J., Agrin, N., Leszyk, J. & Witman, G. B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103–113 (2005).
Nicastro, D. et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944–948 (2006).
Gui, L. et al. Scaffold subunits support associated subunit assembly in the Chlamydomonas ciliary nexin–dynein regulatory complex. Proc. Natl Acad. Sci. USA 116, 23152–23162 (2019).
Urbanska, P. et al. The CSC proteins FAP61 and FAP251 build the basal substructures of radial spoke 3 in cilia. Mol. Biol. Cell 26, 1463–1475 (2015).
Fu, G. et al. The I1 dynein-associated tether and tether head complex is a conserved regulator of ciliary motility. Mol. Biol. Cell 29, 1048–1059 (2018).
Fu, G. et al. Structural organization of the C1a–e–c supercomplex within the ciliary central apparatus. J. Cell Biol. 218, 4236–4251 (2019).
Oda, T., Abe, T., Yanagisawa, H. & Kikkawa, M. Structure and function of outer dynein arm intermediate and light chain complex. Mol. Biol. Cell 27, 1051–1059 (2016).
Yamamoto, R., Hwang, J., Ishikawa, T., Kon, T. & Sale, W. S. Composition and function of ciliary inner-dynein-arm subunits studied in Chlamydomonas reinhardtii. Cytoskeleton (Hoboken) 78, 77–96 (2021).
Pigino, G. et al. Cryoelectron tomography of radial spokes in cilia and flagella. J. Cell Biol. 195, 673–687 (2011).
Oda, T., Yanagisawa, H., Kamiya, R. & Kikkawa, M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 346, 857–860 (2014).
Walton, T., Wu, H. & Brown, A. Structure of a microtubule-bound axonemal dynein. Nat. Commun. 12, 477 (2021).
Ghanaeian, A. et al. Integrated modeling of the nexin–dynein regulatory complex reveals its regulatory mechanism. Nat. Commun. 14, 5741 (2023).
Walton, T. et al. Axonemal structures reveal mechanoregulatory and disease mechanisms. Nature 618, 625–633 (2023).
Gui, M. et al. Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 28, 29–37 (2021).
Meng, X. et al. Multi-scale structures of the mammalian radial spoke and divergence of axonemal complexes in ependymal cilia. Nat. Commun. 15, 362 (2024).
Lin, J. et al. Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia. Nat. Commun. 5, 5727 (2014).
Zhao, Y. et al. Structural insights into the cause of human RSPH4A primary ciliary dyskinesia. Mol. Biol. Cell 32, 1202–1209 (2021).
Hwang, J. Y. et al. LRRC23 truncation impairs radial spoke 3 head assembly and sperm motility underlying male infertility. eLife 12, RP90095 (2023).
Li, Y. et al. LRRC23 deficiency causes male infertility with idiopathic asthenozoospermia by disrupting the assembly of radial spokes. Clin. Genet 104, 694–699 (2023).
Li, W. et al. Biallelic mutations of CFAP251 cause sperm flagellar defects and human male infertility. J. Hum. Genet 64, 49–54 (2019).
Liu, S. et al. CFAP61 is required for sperm flagellum formation and male fertility in human and mouse. Development 148, dev199805 (2021).
Martinez, G. et al. Biallelic variants in MAATS1 encoding CFAP91, a calmodulin-associated and spoke-associated complex protein, cause severe astheno-teratozoospermia and male infertility. J. Med Genet 57, 708–716 (2020).
Dymek, E. E. & Smith, E. F. A conserved CaM- and radial spoke associated complex mediates regulation of flagellar dynein activity. J. Cell Biol. 179, 515–526 (2007).
Oda, T., Yanagisawa, H., Yagi, T. & Kikkawa, M. Mechanosignaling between central apparatus and radial spokes controls axonemal dynein activity. J. Cell Biol. 204, 807–819 (2014).
Patel-King, R. S., Gorbatyuk, O., Takebe, S. & King, S. M. Flagellar radial spokes contain a Ca2+-stimulated nucleoside diphosphate kinase. Mol. Biol. Cell 15, 3891–3902 (2004).
Grossman-Haham, I. et al. Structure of the radial spoke head and insights into its role in mechanoregulation of ciliary beating. Nat. Struct. Mol. Biol. 28, 20–28 (2021).
Smith, E. F. & Yang, P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton 57, 8–17 (2004).
Dymek, E. E., Heuser, T., Nicastro, D. & Smith, E. F. The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol. Biol. Cell 22, 2520–2531 (2011).
Barber, C. F., Heuser, T., Carbajal-Gonzalez, B. I., Botchkarev, V. V. Jr. & Nicastro, D. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol. Biol. Cell 23, 111–120 (2012).
Yang, P. et al. Radial spoke proteins of Chlamydomonas flagella. J. Cell Sci. 119, 1165–1174 (2006).
Heuser, T., Dymek, E. E., Lin, J., Smith, E. F. & Nicastro, D. The CSC connects three major axonemal complexes involved in dynein regulation. Mol. Biol. Cell 23, 3143–3155 (2012).
King, S. M., Otter, T. & Witman, G. B. Purification and characterization of Chlamydomonas flagellar dyneins. Methods Enzymol. 134, 291–306 (1986).
Yang, P., Diener, D. R., Rosenbaum, J. L. & Sale, W. S. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J. Cell Biol. 153, 1315–1326 (2001).
Satouh, Y. & Inaba, K. Proteomic characterization of sperm radial spokes identifies a novel spoke protein with an ubiquitin domain. FEBS Lett. 583, 2201–2207 (2009).
Fujisawa, K.Regulation of adenine nucleotide metabolism by adenylate kinase isozymes: physiological roles and diseases. Int. J. Mol. Sci. 24, 5561 (2023).
Fernandez-Gonzalez, A., Kourembanas, S., Wyatt, T. A. & Mitsialis, S. A. Mutation of murine adenylate kinase 7 underlies a primary ciliary dyskinesia phenotype. Am. J. Respir. Cell Mol. Biol. 40, 305–313 (2009).
Lores, P. et al. Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum. Mol. Genet 27, 1196–1211 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Wriggers, W. Conventions and workflows for using Situs. Acta Crystallogr. D Biol. Crystallogr. 68, 344–351 (2012).
Chen, Z. et al. De novo protein identification in mammalian sperm using in situ cryoelectron tomography and AlphaFold2 docking. Cell 186, 5041–5053 (2023).
Zhang, S., Liu, N., Li, W. & Yan, S. Umbrella structure building design method via case-based design and statistical analysis of structural morphological parameters. J. Build. Eng. 45, 103542 (2022).
Musrati, R. A., Kollarova, M., Mernik, N. & Mikulasova, D. Malate dehydrogenase: distribution, function and properties. Gen. Physiol. Biophys. 17, 193–210 (1998).
Chen, Y. et al. STYXL1 regulates CCT complex assembly and flagellar tubulin folding in sperm formation. Nat. Commun. 15, 44 (2024).
Isrie, M. et al. Homozygous missense mutation in STYXL1 associated with moderate intellectual disability, epilepsy and behavioural complexities. Eur. J. Med Genet 58, 205–210 (2015).
Kultgen, P. L., Byrd, S. K., Ostrowski, L. E. & Milgram, S. L. Characterization of an A-kinase anchoring protein in human ciliary axonemes. Mol. Biol. Cell 13, 4156–4166 (2002).
Bontems, F. et al. C2orf62 and TTC17 are involved in actin organization and ciliogenesis in zebrafish and human. PLoS ONE 9, e86476 (2014).
Brody, S. L. et al. Undocking of an extensive ciliary network induces proteostasis and cell fate switching resulting in severe primary ciliary dyskinesia. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.adp5173 (2024).
Lin, J. & Nicastro, D.Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, eaar1968 (2018).
Yamamoto, R. et al. The MIA complex is a conserved and novel dynein regulator essential for normal ciliary motility. J. Cell Biol. 201, 263–278 (2013).
DiPetrillo, C. G. & Smith, E. F. Pcdp1 is a central apparatus protein that binds Ca2+-calmodulin and regulates ciliary motility. J. Cell Biol. 189, 601–612 (2010).
Yagi, T. ADP-dependent microtubule translocation by flagellar inner-arm dyneins. Cell Struct. Funct. 25, 263–267 (2000).
Wirschell, M. et al. Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch. Biochem. Biophys. 510, 93–100 (2011).
Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 69, 401–422 (2007).
Lin, J. et al. Building blocks of the nexin–dynein regulatory complex in Chlamydomonas flagella. J. Biol. Chem. 286, 29175–29191 (2011).
Paudel, B. et al. Sperm capacitation is associated with phosphorylation of the testis-specific radial spoke protein Rsph6a. Biol. Reprod. 100, 440–454 (2019).
Porter, M. E. & Sale, W. S. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151, F37–F42 (2000).
Habermacher, G. & Sale, W. S. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J. Cell Biol. 136, 167–176 (1997).
King, S. J. & Dutcher, S. K. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J. Cell Biol. 136, 177–191 (1997).
Yang, P. & Sale, W. S. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J. Biol. Chem. 275, 18905–18912 (2000).
Gokhale, A., Wirschell, M. & Sale, W. S. Regulation of dynein-driven microtubule sliding by the axonemal protein kinase CK1 in Chlamydomonas flagella. J. Cell Biol. 186, 817–824 (2009).
Gui, M., Wang, X., Dutcher, S. K., Brown, A. & Zhang, R. Ciliary central apparatus structure reveals mechanisms of microtubule patterning. Nat. Struct. Mol. Biol. 29, 483–492 (2022).
Antunes, M. B. & Cohen, N. A. Respiratory cilia: principles of mucociliary clearance. In Rhinology and Facial Plastic Surgery (eds Stucker, F. J. et al.) (Springer, 2009).
Klumpp, S., Cohen, P. & Schultz, J. E.Okadaic acid, an inhibitor of protein phosphatase 1 in Paramecium, causes sustained Ca2+-dependent backward swimming in response to depolarizing stimuli. EMBO J. 9, 685–689 (1990).
Christensen, S. T. et al. A regulatory light chain of ciliary outer arm dynein in Tetrahymena thermophila. J. Biol. Chem. 276, 20048–20054 (2001).
Kinoshita, S., Miki-Noumura, T. & Omoto, C. K. Regulatory role of nucleotides in axonemal function. Cell Motil. Cytoskeleton 32, 46–54 (1995).
Sakato-Antoku, M. & King, S. M. Developmental changes in ciliary composition during gametogenesis in Chlamydomonas. Mol. Biol. Cell 33, br10 (2022).
Han, L. et al. Cryo-EM structure of an active central apparatus. Nat. Struct. Mol. Biol. 29, 472–482 (2022).
Wu, H. et al. Adenylate kinase phosphate energy shuttle underlies energetic communication in flagellar axonemes. Sci. China Life Sci. 67, 1697–1714 (2024).
Ma, M. et al. Structure of the decorated ciliary doublet microtubule. Cell 179, 909–922 (2019).
Leung, M. R. et al. Structural diversity of axonemes across mammalian motile cilia. Nature 637, 1170–1177 (2025).
Yin, W. et al. Mice with a deletion of Rsph1 exhibit a low level of mucociliary clearance and develop a primary ciliary dyskinesia phenotype. Am. J. Respir. Cell Mol. Biol. 61, 312–321 (2019).
Yoke, H. et al. Rsph4a is essential for the triplet radial spoke head assembly of the mouse motile cilia. PLoS Genet. 16, e1008664 (2020).
Zariwala, M. A., Knowles, M. R. & Leigh, M. W. Primary ciliary dyskinesia. In GeneReviews (eds Adam, M. P. et al.) (University of Washington, 1993).
Xiang, M. et al. A novel homozygous missense mutation in AK7 causes multiple morphological anomalies of the flagella and oligoasthenoteratozoospermia. J. Assist. Reprod. Genet. 39, 261–266 (2022).
Mata, M. et al. New adenylate kinase 7 (AK7) mutation in primary ciliary dyskinesia. Am. J. Rhinol. Allergy 26, 260–264 (2012).
Milara, J., Armengot, M., Mata, M., Morcillo, E. J. & Cortijo, J. Role of adenylate kinase type 7 expression on cilia motility: possible link in primary ciliary dyskinesia. Am. J. Rhinol. Allergy 24, 181–185 (2010).
Sha, Y. et al. Deficiency in AK9 causes asthenozoospermia and male infertility by destabilising sperm nucleotide homeostasis. EBioMedicine 96, 104798 (2023).
Yang, H. W. et al. A role for mutations in AK9 and other genes affecting ependymal cells in idiopathic normal pressure hydrocephalus. Proc. Natl Acad. Sci. USA 120, e2300681120 (2023).
Broeks, M. H. et al. MDH1 deficiency is a metabolic disorder of the malate-aspartate shuttle associated with early onset severe encephalopathy. Hum. Genet. 138, 1247–1257 (2019).
Arafat, M. et al. Mutation in CATIP (C2orf62) causes oligoteratoasthenozoospermia by affecting actin dynamics. J. Med. Genet. 2019, 106825 (2020).
Ruland, L. et al. Organoid models of fibrolamellar carcinoma mutations reveal hepatocyte transdifferentiation through cooperative BAP1 and PRKAR2A loss. Nat. Commun. 14, 2377 (2023).
Cela, P. et al. MORN5 expression during craniofacial development and its interaction with the BMP and TGFβ pathways. Front. Physiol. 7, 378 (2016).
Hu, T. et al. Biallelic CFAP61 variants cause male infertility in humans and mice with severe oligoasthenoteratozoospermia. J. Med. Genet. 60, 144–153 (2023).
Ma, A. et al. Biallelic variants in CFAP61 cause multiple morphological abnormalities of the flagella and male infertility. Front. Cell Dev. Biol. 9, 803818 (2021).
Kherraf, Z. E. et al. A homozygous ancestral SVA-insertion-mediated deletion in WDR66 induces multiple morphological abnormalities of the sperm flagellum and male infertility. Am. J. Hum. Genet. 103, 400–412 (2018).
Auguste, Y. et al. Loss of calmodulin- and radial-spoke-associated complex protein CFAP251 leads to immotile spermatozoa lacking mitochondria and infertility in men. Am. J. Hum. Genet. 103, 413–420 (2018).
Wloga, D. et al. Glutamylation on alpha-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena thermophila. Eukaryot. Cell 7, 1362–1372 (2008).
Chittum, H. S. et al. Rabbit β-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry 37, 10866–10870 (1998).
Schoff, P. K., Cheetham, J. & Lardy, H. A. Adenylate kinase activity in ejaculated bovine sperm flagella. J. Biol. Chem. 264, 6086–6091 (1989).
Kinukawa, M. & Vacquier, V. D. Adenylate kinase in sea urchin embryonic cilia. Cell Motil. Cytoskeleton 64, 310–319 (2007).
Iancu, C. V. et al. Electron cryotomography sample preparation using the Vitrobot. Nat. Protoc. 1, 2813–2819 (2006).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Heumann, J. M., Hoenger, A. & Mastronarde, D. N. Clustering and variance maps for cryo-electron tomography using wedge-masked differences. J. Struct. Biol. 175, 288–299 (2011).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Kimanius, D. et al. Data-driven regularization lowers the size barrier of cryo-EM structure determination. Nat. Methods 21, 1216–1221 (2024).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2021).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in PHENIX. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
We thank C. Xu for providing EM training and management of EM instrumentation in the Louise Mashal Gabbay Cellular Visualization Facility at Brandeis University. We thank D. Stoddard and J. Martinez Diaz for providing EM training and management of the UT Southwestern Medical Center cryo-EM facility (funded in part by Cancer Prevention and Research Institute of Texas (CPRIT) Core Facility Awards RP170644 and RP220582). We thank D. Mastronarde and J. Heumann (University of Colorado at Boulder) for continued development of image-processing tools, including PEET classification. This research benefitted from the computational resources provided by the BioHPC high-performance computing facility located in the Lyda Hill Department of Bioinformatics at UT Southwestern Medical Center. Mass spectroscopy was conducted in the UT Southwestern Proteomics Core Facility. We thank M. Porter (University of Minnesota), S. King (University of Connecticut), J. R. McIntosh (University of Colorado), B. Tu (UT Southwestern Medical Center) and J. Pinskey (University of Massachusetts) for their critical review and helpful feedback on the paper. This work was supported by funding by National Institutes of Health and CPRIT grants R01GM083122 and RR140082 to D.N. and R35GM130289 to X.Z.
Author information
Authors and Affiliations
Contributions
Y.Z. performed sample preparation, data collection, data processing for the cryo-EM study of mouse, sample preparation and analysis for the LC–MS of mouse and figure and video preparation. K.S. performed sample preparation, data collection, data processing for the cryo-ET study of mouse and sample preparation and analysis for the LC–MS and enzyme activity assay of Tetrahymena. L.G. performed data collection for the cryo-EM study of mouse. A.T.T. performed data analysis for the LC–MS of mouse. A.F.G., S.Z., P.P.D. and S.A.M. generated and provided the AK7−/− and AK1−/− mouse strains. X.Z. performed data processing and modeling for the cryo-EM study of mouse. Y.Z., X.Z. and D.N. wrote the paper. D.N. conceptualized and directed the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Lea Alford, Mingxi Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Structural comparison of the 96-nm axonemal repeat between WT and mutant axonemes from Tetrahymena (a-f) and mouse respiratory (g-x) cilia.
a-c) Tomographic slices (a-c) and an isosurface rendering (d) of the averaged 96-nm axonemal repeat show the full-length RS3 in Tetrahymena WT (a, d), whereas class-averaging of a CSC-mutant (CFAP61-KO) (b, c) reveals that large parts of RS3 are missing from about one third of the repeats (c) (based on data previously published7). The CSC subunits CFAP61 (green) and CFAP251 (red) are colored at the base of RS3 (d). e, f) Negative staining EM of Tetrahymena WT (e) and CFAP61-KO (f) axonemes after mild salt-extraction (0.6 M NaCl) shows in WT three RS per 96-nm repeat (from distal to proximal: RS3/orange, RS2/blue and RS1/green), whereas in CFAP61-KO RS3 is missing. g-r) Tomographic slices (g-i, k-m, o-q) and isosurface renderings (j, n, r) of the averaged 96-nm repeats from WT (g-j), AK1−/− (k-n), and AK7−/− (o-r) mouse respiratory cilia show RS3 in cross-sectional view (g, k, o) and in longitudinal view (h, l, p), and the RS bases in longitudinal bottom view, similar to the isosurface renderings. White arrowheads indicate missing structures in the mutants, that is the missing distal part of RS3 in AK7−/− (o, p), and a protrusion from the DMT wall between RS1 and RS2 (red arrowheads and colored red in j and r) that is missing in AK1−/− (m, n). s-x) Tomographic slices of class averages of RS3 from AK7−/− axonemes show RS3 in the same orientations as in (o-p). The classification analysis revealed that all AK7−/− repeats have truncated RS3, but with slightly different levels of truncation (indicated by white arrowheads), from the shortest (class 1) to the longer one (class 3). The percentage of subtomograms included in each class average is indicated. Note that, the distal side of the cilium on the right in (a-d), otherwise, cross-sections are viewed from distal to proximal orientation, and in longitudinal views with distal side of the cilium on the left. Scale bars: (a-c) 20 nm, (e-f) 100 nm, (g-i, k-m, o-q) 20 nm, (s-x) 20 nm.
Extended Data Fig. 2 Comparative proteomic analysis of Tetrahymena WT and CFAP61-KO axonemes, and WT and AK7−/− mouse respiratory axonemes.
a) In the plot, each protein is mapped according to its number of peptide spectra detected in high-salt extracted WT (EXWT) sample on the x-axis, and in high-salt extracted CFAP61-KO (EXCFAP61-KO) sample on the y-axis. Proteins with a ratio higher than 0.5 are shown as gray dots, and proteins below a ratio of 0.5 (and number of peptide spectra in EXWT >5) are shown as purple dots or red dots, with red indicating conservation between Tetrahymena and mouse (additional information in Supplementary Table 1). The ratios for CFAP91 and CFAP251 (blue dots) were above 0.5, consistent with them being previously known RS3 base proteins. b) In the volcano plot, each protein is represented as a circle (identified in all three experimental repeats) or triangle (identified in two repeats) and is mapped according to its average fold change in AK7−/− compared to WT axonemes on the x-axis, and the p value (calculated using one-tailed t-test, exact p values are provided in the source data) on the y-axis. The protein abundances of AK7, AK9, LRRC23, MDH1B and AKAP14 were significantly reduced (red dots). Note that C10ORF53 was also considerably reduced (ratio, 0.07) in one of three LC-MS experiments, but it was not plotted here, because in the other one experiment its abundance (even in WT) was too low for quantification. Seven additional proteins with low abundance in AK7−/− compared to WT axonemes (ratio <0.1) are labeled with either T# and D# (identified in three and two experimental repeats respectively, and the number represents the row in the corresponding source data table), and comments are added to the source data table to explain reasons for excluding them as RS3 candidates. Proteins on the left of the ratio <0.5 were reduced by up to 50% in AK7−/−. The protein abundances of MDH1, PRKAR2A, CATIP, MORN5, STYXL1 were mildly reduced (blue dots). AK1 and AK8, which previously co-IP-ed with the RS3 proteins CFAP251 and CFAP617, but showed here similar abundance between the mutant and WT (green dots).
Extended Data Fig. 3 Sample preparation and cryo-EM data processing part I (using cryoSPARC) to determine the 3D structure of the DMT and RS3 in mouse respiratory cilia.
a) Samples for cryo-EM single particle analysis were prepared by excising the trachea from WT mice, using calcium-shock and detergent treatment to remove the ciliary axonemes from the multiciliated respiratory epithelium, and splaying the axonemes into DMTs using ATP and mild salt-treatment. The DMTs were then plunge-frozen, cryo-EM images were recorded in a semi-automated fashion on a Titan Krios, and after image processing (see b) the 3D structure of the DMT repeat was reconstructed. shows the image processing steps (part I) for cryo-EM single particle analysis using the software cryoSPARC99. After DMT signal subtraction, the micrographs were importing into Relion102 for the 3D reconstruction of RS3 (see image processing part II, Extended Data Fig. 4). c) Example of a motion-corrected cryo-EM micrograph of a DMT with regularly spaced RS3 (arrowheads), the characteristic spacing allowed manual particle picking of RS3; attempts of automatic and AI-assisted particle picking failed. This experiment was repeated 50,000 times independently with similar results. d) Examples of 2D class averages centered on the docking site of RS3 to the DMT. e, f) Cross-sectional (left) and longitudinal (right) slices (e) and isosurface renderings (f) of the 3D reconstructed DMT with local resolution distribution indicated as color-gradient (estimated by cryoSPARC99). Details of the DMT are well-resolved, such as the tektin-MIPs in the A-tubule (arrowhead in e). However, the alignment is dominated by the DMT and the RS3 structure is blurred (arrows in e). Scale bars: (a, c) 50 nm, (d, e) 20 nm.
Extended Data Fig. 4 Cryo-EM image processing part II (using Relion) to determine the 3D structure of RS3 in mouse respiratory cilia.
After blush refinement102 the full-length RS3 structure could be resolved, but with low resolution in the head-neck-stalk region (top right). Separate processing (for example, signal subtraction, blush refinement and 3D classification) of the head-neck and base-stalk regions improved the quality of both maps to a resolution of 7.1 Å and 4.7 Å, respectively (shown in Extended Data Fig. 5a). Each step uses the results of the preceding step as a direct starting point, including references for 3D refinement and classification. The composite map of the full-length RS3 was generated by aligning the two maps based on their overlapping region in ChimeraX100.
Extended Data Fig. 5 FSC curves and atomic model of RS3 in mouse respiratory cilia.
a) Gold-standard FSC (Fourier shell correlation) curves of the head-neck and base-stalk regions were estimated by Relion102. The 7.1-Å cryo-EM map of the RS3 head-neck-stalk regions clearly shows most of the protein secondary structures, but it does not resolve amino acid sidechains. The resolution of the RS3 atomic model was estimated as 6.3 Å by FSC (threshold 0.5) between the model and the composite map. b) The AlphaFold2-predicted and refined atomic models of the RS3 proteins and RS3-associated proteins are individually labelled. Subunit coloring following the color scheme established in Fig. 2. c, d) Examples of sidechain fitting to the EM density in the RS3 base region.
Extended Data Fig. 6 The AlphaFold2 multimer model predictions and fitting to the RS3 head-neck region.
a–d) Each row shows a complex prediction between of two RS3 proteins as labelled and colored in the second column, that is two catalytic domains of AK9 with LRRC23s (a, b), the C-terminal fragments of AK7 and AK9 (c), and the C-terminal fragment of AK9 with the N-terminal fragment of MDH1B (d). The first column shows the AlphaFold2 multimer model predictions103,104 color-coded by ‘per-residue confidence score’ (pLDDT) from the highest (blue) to the lowest score (red). Note that the confidence scores are mostly high at the predicted protein interfaces. The second and third columns show the predicted AlphaFold2 multimer model fitted into our cryo-EM map without (2nd column) and with refinement (3rd column); note that other RS3 proteins are not shown in the cryo-EM map for clarity; black arrowheads highlight some of the larger refinements. Detailed fitting and refinement of the individual residues into the density was carried out with secondary structure restraints, and was further refined for coordinates and b-factor with secondary structure and Ramachandran restraints106. The fourth column shows a zoomed-out overview with the region shown in the third column indicated by a box. a’-d’) Predicted aligned error (PAE) plot showing low (blue) to high (red) alignment error between chains for the corresponding predictions in panels a-d.
Extended Data Fig. 7 The AlphaFold2 multimer model predictions and fitting to the RS3 neck-stalk region.
a–d) Each row shows a complex prediction between two to four RS3 proteins as labelled and colored in the second column, that is between the full length MDH1 and MDH1B, that contains an extra N-terminal domain (bottom) (a), the N-terminal fragment of MDH1B with C10ORF53 (b), AKAP14 with fragments of CATIP, CFAP91, and PRKAR2A (c), and a fragment of CFAP91 with STYXL1 and MORN5 (d). The first column shows the AlphaFold2 multimer model predictions103,104 color-coded by ‘per-residue confidence score’ (pLDDT) from the highest (blue) to the lowest score (red). Note that the confidence scores are mostly high at the predicted protein interfaces. The second and third columns show the predicted AlphaFold2 multimer model fitted into our cryo-EM map without (2nd column) and with refinement (3rd column); note that other RS3 proteins are not shown in the cryo-EM map for clarity; black arrowheads highlight some of the larger refinements. Detailed fitting and refinement of the individual residues into the density was carried out with secondary structure restraints, and was further refined for coordinates and b-factor with secondary structure and Ramachandran restraints106. The fourth column shows a zoomed-out overview with the region shown in the third column indicated by a box. a’-d’) Predicted aligned error (PAE) plot showing low (blue) to high (red) alignment error between chains for the corresponding predictions in panels a-d. Noted that the color codes of pLDDT and PAE are shown in Extended Data Fig. 6.
Extended Data Fig. 8 PRKAR2A and CATIP are anchored in the RS3 stalk, but their functional domains are flexibly positioned.
a) Slice through the cryo-EM 3D reconstructed RS3 head-neck. Note the blurred and unresolved density on the proximal side of the neck region (ellipse), suggesting structural flexibility of subunit(s) in this region. b, c) AlpaFold2 model predictions of full length PRKAR2A and CATIP show both two high confidence regions (blue), that is, large functional domains that connect through flexible linkers to small helical segments (that is the N-terminal region of PRKAR2A and mid region of CATIP) that attach the proteins to the RS3 stalk by interacting with CFAP91 and each other. The model predictions are color-coded by ‘per-residue confidence score’ (pLDDT) from the highest (blue) to the lowest score (red). d, e) The unassigned, large globular domains of the AlphaFold2 predicted models of PRKAR2A and CATIP show reasonable fitting to the unresolved cryo-EM density in the RS3 neck region. (e) shows a zoom-in of the dash-circled area in (d). f, g) The atomic model of RS3 (as seen in Fig. 2) but including the full-length predicted models of PRKAR2A and CATIP. h, i) The long, disordered liker regions of CATIP (h) and PRKAR2A (i) could provide a large radius of gyration (symbolized as sphere) of the large globular domains around their stalk-anchored helical domains (maximum radius predicted based on number of amino acids in the linker x 3.5 Å). Scale bar: (a) 10 nm.
Extended Data Fig. 9 Similar architectural principles of RSs in mouse respiratory cilia and Chlamydomonas cilia suggest suitability for mechanical signal transduction.
a) In the mouse RS3, the long α-helices of two AK7 molecules interact (circled) with the hooked end of the long α-helix of CFAP91. Two additional struts (AK9 and MDH1/MDH1B) in the neck region, support the RS3 head. CFAP91 continues from the transition between neck and stalk to the RS3 base, completing the ‘fixed umbrella architecture’ from head to base. b) In Chlamydomonas RS2 (and RS1), the α-helices of two RSP3 and two RSP2 molecules form four struts that interact (circled) at the transition between neck and stalk. The two RSP3 molecules continue as scaffold through the stalk and based, completing also a ‘fixed umbrella architecture’. The RSs are viewed with the distal side of the cilium on the left. Our RS3 model was combined with the DMT atomic model of human (8J07) for comparison with the Chlamydomonas RS2 and DMT (8GLV).
Extended Data Fig. 10 Cryo-ET of Primary Ciliary Dyskinesia (PCD)-causing radial-spoke mutants of human (b-d) and mouse (e-f) respiratory cilia.
a) Cartoon representations of typical WT [9 + 2] cilia and mutant cilia with various CPC and DMT defects (viewed from distal to proximal). b–d) Cross-sectional tomographic slices through individual representative reconstruction of human WT cilia (b, n = 12, using data previously published19), human RSPH1−/− cilia (c, n = 20, using data previously published19) and human RSPH4A−/− cilia (d, n = 13, using data previously published20). Both RSPH1−/− and RSPH4A−/− cause PCD with primary structural defects in RS1 and RS2 head and secondary defects in the CPC19,20. e, f) Cross-sectional tomographic slices through individual representative reconstruction of mouse WT cilia (e, n = 14) and AK7−/− cilia (f, n = 13), which causes PCD and RS3 structural defects. Numbers represent the ratio of the respective phenotype. Note that despite the severe PCD symptoms of homozygous AK7−/− mice, the CPC phenotype resembles that in WT, in contrast to the RS1/2 human ciliopathy patients. Scale bar: (b-f) 50 nm.
Supplementary information
Supplementary Information
Supplementary Tables 1–4 and References.
Supplementary Video 1
Rotation of cryo-ET and cryo-EM reconstructions and the atomic model of full-length RS3 from mouse respiratory cilia. First, a 3D isosurface rendering of the subtomogram-averaged 96-nm axonemal repeat from mouse respiratory cilia is rotated into a longitudinal back view (with proximal on the left), revealing the arrangement of ciliary complexes, including RS3 (colored orange). The animation blends to the cryo-EM single-particle reconstruction of RS3 with a resolution good enough to discern secondary structure features. The animation starts with a gray isosurface rendering that is then colored according to the fitted subunits, following the color scheme established in Fig. 2. RS3 is rotated 90° (into cross-sectional view of the DMT seen from distal) and the AlphaFold2-predicted atomic models (fitted and refined) are placed sequentially from the base to the head. The RS3 atomic model is rotated around the y axis and into a view facing the RS3 surface that interacts with the CPC projections. The AK catalytic domains are highlighted. Then, RS3 is rotated back and the animation ends with our RS3 atomic model integrated in the model of the 96-nm axonemal repeat of mammalian respiratory cilia16.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed negative-staining images.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Song, K., Tavakoli, A. et al. Mouse radial spoke 3 is a metabolic and regulatory hub in cilia. Nat Struct Mol Biol 32, 1542–1554 (2025). https://doi.org/10.1038/s41594-025-01594-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01594-6
This article is cited by
-
A metabolic hub in ciliary and flagellar motion
Nature Structural & Molecular Biology (2025)
-
Heterogeneity of radial spoke components in Tetrahymena cilia
Cellular and Molecular Life Sciences (2025)