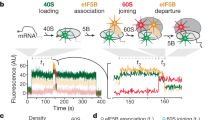
Abstract
Human translation initiation requires accurate recognition of translation start sites. While AUG codons are canonical start sites, non-AUG codons are also used, typically with lower efficiency. The initiator tRNA and initiation factors eIF1 and eIF5 control recognition. How they distinguish different start sites yet allow flexible recognition remains unclear. Here we used real-time single-molecule assays and an in vitro reconstituted human system to reveal how eIF1 and eIF5 direct start site selection. eIF1 binds initiation complexes in two modes: stable binding during scanning, followed by transient, concentration-dependent rebinding after start site recognition. Termination of eIF1 rebinding requires transient and concentration-dependent binding by eIF5, which allows the formation of translation competent ribosomes. Non-AUG start sites differentially stabilize eIF1 and destabilize eIF5 binding, blocking initiation at multiple points. We confirmed these opposing effects in human cells. Collectively, our findings uncover that eIF1 and eIF5 directly compete to bind initiation complexes and illuminate how their dynamic interplay tunes the fidelity of start site recognition, which has broad connections to health and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Processed single-molecule data and source data needed to recapitulate single-molecule figure plots throughout the manuscript are available from GitHub (https://github.com/LapointeLab/eIF1-eIF5-2024-paper). Specific requests can be requested using the issue feature or by email request to C.P.L. Structure images were generated using published models (PDB 6ZMW and PDB 8OZ0) and ChimeraX-1.71.1 software. Source data are provided with this paper.
Code availability
All code needed to analyze the single-molecule data is available on GitHub (https://github.com/LapointeLab/eIF1-eIF5-2024-paper).
References
Kozak, M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol. Cell. Biol. 9, 5073–5080 (1989).
Peabody, D. S. Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem. 264, 5031–5035 (1989).
Kearse, M. G. & Wilusz, J. E. Non-AUG translation: a new start for protein synthesis in eukaryotes. Genes Dev. 31, 1717–1731 (2017).
Andreev, D. E. et al. Non-AUG translation initiation in mammals. Genome Biol. 23, 111 (2022).
Dever, T. E., Ivanov, I. P. & Hinnebusch, A. G. Translational regulation by uORFs and start codon selection stringency. Genes Dev. 37, 474–489 (2023).
Sokabe, M. & Fraser, C. S. Toward a kinetic understanding of eukaryotic translation. Cold Spring Harb. Perspect. Biol. 11, a032706 (2018).
Jackson, R. J., Hellen, C. U. T. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Aitken, C. E. & Lorsch, J. R. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 19, 568–576 (2012).
Hinnebusch, A. G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 83, 779–812 (2014).
Hinnebusch, A. G. Structural insights into the mechanism of scanning and start codon recognition in eukaryotic translation initiation. Trends Biochem. Sci. 42, 589–611 (2017).
Wang, J. et al. Rapid 40S scanning and its regulation by mRNA structure during eukaryotic translation initiation. Cell 185, 4474–4487 (2022).
Kozak, M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44, 283–292 (1986).
Hashem, Y. & Frank, J. The jigsaw puzzle of mRNA translation initiation in eukaryotes: a decade of structures unraveling the mechanics of the process. Annu. Rev. Biophys. 47, 125–151 (2018).
Guca, E. & Hashem, Y. Major structural rearrangements of the canonical eukaryotic translation initiation complex. Curr. Opin. Struct. Biol. 53, 151–158 (2018).
Brito Querido, J., Díaz-López, I. & Ramakrishnan, V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat. Rev. Mol. Cell Biol. 25, 168–186 (2024).
Algire, M. A., Maag, D. & Lorsch, J. R. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20, 251–262 (2005).
Saini, A. K. et al. Eukaryotic translation initiation factor eIF5 promotes the accuracy of start codon recognition by regulating Pi release and conformational transitions of the preinitiation complex. Nucleic Acids Res. 42, 9623–9640 (2014).
Das, S., Ghosh, R. & Maitra, U. Eukaryotic translation initiation factor 5 functions as a GTPase-activating protein. J. Biol. Chem. 276, 6720–6726 (2001).
Paulin, F. E., Campbell, L. E., O’Brien, K., Loughlin, J. & Proud, C. G. Eukaryotic translation initiation factor 5 (eIF5) acts as a classical GTPase-activator protein. Curr. Biol. 11, 55–59 (2001).
Pestova, T. V. et al. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 403, 332–335 (2000).
Wang, J. et al. eIF5B gates the transition from translation initiation to elongation. Nature 573, 605–608 (2019).
Brown, Z. P. et al. Molecular architecture of 40S translation initiation complexes on the hepatitis C virus IRES. EMBO J. 41, e110581 (2022).
Kazan, R. et al. Role of aIF5B in archaeal translation initiation. Nucleic Acids Res. 50, 6532–6548 (2022).
Lapointe, C. P. et al. eIF5B and eIF1A reorient initiator tRNA to allow ribosomal subunit joining. Nature 607, 185–190 (2022).
Maag, D., Fekete, C. A., Gryczynski, Z. & Lorsch, J. R. A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol. cell 17, 265–275 (2005).
Cheung, Y.-N. et al. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev. 21, 1217–1230 (2007).
Nanda, J. S. et al. eIF1 controls multiple steps in start codon recognition during eukaryotic translation initiation. J. Mol. Biol. 394, 268–285 (2009).
Nanda, J. S., Saini, A. K., Muñoz, A. M., Hinnebusch, A. G. & Lorsch, J. R. Coordinated movements of eukaryotic translation initiation factors eIF1, eIF1A, and eIF5 trigger phosphate release from eIF2 in response to start codon recognition by the ribosomal preinitiation complex. J. Biol. Chem. 288, 5316–5329 (2013).
Martin-Marcos, P. et al. β-Hairpin loop of eukaryotic initiation factor 1 (eIF1) mediates 40 S ribosome binding to regulate initiator tRNA(Met) recruitment and accuracy of AUG selection in vivo. J. Biol. Chem. 288, 27546–27562 (2013).
Martin-Marcos, P. et al. Enhanced eIF1 binding to the 40S ribosome impedes conformational rearrangements of the preinitiation complex and elevates initiation accuracy. RNA 20, 150–167 (2014).
Zhou, F., Zhang, H., Kulkarni, S. D., Lorsch, J. R. & Hinnebusch, A. G. eIF1 discriminates against suboptimal initiation sites to prevent excessive uORF translation genome-wide. RNA 26, 419 (2020).
Donahue, T. F. & Cigan, A. M. Genetic selection for mutations that reduce or abolish ribosomal recognition of the HIS4 translational initiator region. Mol. Cell. Biol. 8, 2955–2963 (1988).
Castilho-Valavicius, B., Yoon, H. & Donahue, T. F. Genetic characterization of the Saccharomyces cerevisiae translational initiation suppressors Sui1, Sui2 and Sui3 and their effects on His4 expression. Genetics 124, 483–495 (1990).
Huang, H., Yoon, H., Hannig, E. M. & Donahue, T. F. GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae. Genes Dev. 11, 2396–2413 (1997).
Fijalkowska, D. et al. eIF1 modulates the recognition of suboptimal translation initiation sites and steers gene expression via uORFs. Nucleic Acids Res. 45, 7997–8013 (2017).
Pisareva, V. P. & Pisarev, A. V. eIF5 and eIF5B together stimulate 48S initiation complex formation during ribosomal scanning. Nucleic Acids Res. 42, 12052–12069 (2014).
Ivanov, I. P., Loughran, G., Sachs, M. S. & Atkins, J. F. Initiation context modulates autoregulation of eukaryotic translation initiation factor 1 (eIF1). Proc. Natl Acad. Sci. USA 107, 18056–18060 (2010).
Loughran, G., Sachs, M. S., Atkins, J. F. & Ivanov, I. P. Stringency of start codon selection modulates autoregulation of translation initiation factor eIF5. Nucleic Acids Res. 40, 2898–2906 (2012).
Asano, K., Clayton, J., Shalev, A. & Hinnebusch, A. G. A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNAMet is an important translation initiation intermediate in vivo. Genes Dev. 14, 2534–2546 (2000).
Valásek, L. et al. The yeast eIF3 subunits TIF32/a, NIP1/c, and eIF5 make critical connections with the 40S ribosome in vivo. Genes Dev. 17, 786–799 (2003).
Yamamoto, Y. et al. The eukaryotic initiation factor (eIF) 5 HEAT domain mediates multifactor assembly and scanning with distinct interfaces to eIF1, eIF2, eIF3, and eIF4G. Proc. Natl Acad. Sci. USA 102, 16164–16169 (2005).
Sokabe, M. & Fraser, C. S. Human eukaryotic initiation factor 2 (eIF2)–GTP–Met-tRNAi ternary complex and eIF3 stabilize the 43 S preinitiation complex. J. Biol. Chem. 289, 31827–31836 (2014).
Pestova, T. V., Borukhov, S. I. & Hellen, C. U. T. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 394, 854–859 (1998).
Pestova, T. V. & Kolupaeva, V. G. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 16, 2906–2922 (2002).
Lomakin, I. B. & Steitz, T. A. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature 500, 307–311 (2013).
Hussain, T. et al. Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell 159, 597–607 (2014).
Llácer, J. L. et al. Conformational differences between open and closed states of the eukaryotic translation initiation complex. Mol. Cell 59, 399–412 (2015).
Brito Querido, J. et al. Structure of a human 48S translational initiation complex. Science 369, 1220–1227 (2020).
Passmore, L. A. et al. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol. Cell 26, 41–50 (2007).
Weisser, M., Voigts-Hoffmann, F., Rabl, J., Leibundgut, M. & Ban, N. The crystal structure of the eukaryotic 40S ribosomal subunit in complex with eIF1 and eIF1A. Nat. Struct. Mol. Biol. 20, 1015–1017 (2013).
Erzberger, J. P. et al. Molecular architecture of the 40S⋅eIF1⋅eIF3 translation initiation complex. Cell 158, 1123–1135 (2014).
Des Georges, A. et al. Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature 525, 491–495 (2015).
Aylett, C. H. S., Boehringer, D., Erzberger, J. P., Schaefer, T. & Ban, N. Structure of a yeast 40S–eIF1–eIF1A–eIF3–eIF3j initiation complex. Nat. Struct. Mol. Biol. 22, 269–271 (2015).
Kratzat, H. et al. A structural inventory of native ribosomal ABCE1–43S pre‐initiation complexes. EMBO J. 40, e105179 (2021).
Simonetti, A., Guca, E., Bochler, A., Kuhn, L. & Hashem, Y. Structural insights into the mammalian late-stage initiation complexes. Cell Rep. 31, 107497 (2020).
Llácer, J. L. et al. Translational initiation factor eIF5 replaces eIF1 on the 40S ribosomal subunit to promote start-codon recognition. eLife 7, 1–33 (2018).
Brito Querido, J. et al. The structure of a human translation initiation complex reveals two independent roles for the helicase eIF4A. Nat. Struct. Mol. Biol. 31, 455–464 (2024).
Chen, J. et al. High-throughput platform for real-time monitoring of biological processes by multicolor single-molecule fluorescence. Proc. Natl Acad. Sci. USA 111, 664–669 (2014).
Sokabe, M. & Fraser, C. S.A helicase-independent activity of eIF4A in promoting mRNA recruitment to the human ribosome. Proc. Natl Acad. Sci. USA 114, 6304–6309 (2017).
Phan, L. et al. Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol. Cell. Biol. 18, 4935–4946 (1998).
Petrychenko, V. et al. Structural basis for translational control by the human 48S initiation complex. Nat. Struct. Mol. Biol. 32, 62–72 (2025).
Lin, K. Y., Nag, N., Pestova, T. V. & Marintchev, A. Human eIF5 and eIF1A compete for binding to eIF5B. Biochemistry 57, 5910–5920 (2018).
Archer, S. K., Shirokikh, N. E., Beilharz, T. H. & Preiss, T. Dynamics of ribosome scanning and recycling revealed by translation complex profiling. Nature 535, 570–574 (2016).
Wagner, S. et al. Selective translation complex profiling reveals staged initiation and co-translational assembly of initiation factor complexes. Mol. Cell 79, 546–560 (2020).
Terenin, I. M. et al. Sliding of a 43S ribosomal complex from the recognized AUG codon triggered by a delay in eIF2-bound GTP hydrolysis. Nucleic Acids Res. 44, 1882–1893 (2016).
Mao, Y., Jia, L., Dong, L., Shu, X. E. & Qian, S.-B. Start codon-associated ribosomal frameshifting mediates nutrient stress adaptation. Nat. Struct. Mol. Biol. 30, 1816–1825 (2023).
Ly, J. et al. Nuclear release of eIF1 restricts start-codon selection during mitosis. Nature 635, 490–498 (2024).
Fraser, C. S., Berry, K. E., Hershey, J. W. B. & Doudna, J. A. eIF3j is located in the decoding center of the human 40S ribosomal subunit. Mol. Cell 26, 811–819 (2007).
Özeş, A. R., Feoktistova, K., Avanzino, B. C. & Fraser, C. S. Duplex unwinding and ATPase activities of the DEAD-box helicase eIF4A are coupled by eIF4G and eIF4B. J. Mol. Biol. 412, 674–687 (2011).
Feoktistova, K., Tuvshintogs, E., Do, A. & Fraser, C. S. Human eIF4E promotes mRNA restructuring by stimulating eIF4A helicase activity. Proc. Natl Acad. Sci. USA 110, 13339–13344 (2013).
Damoc, E. et al. Structural characterization of the human eukaryotic initiation factor 3 protein complex by mass spectrometry. Mol. Cell. Proteomics 6, 1135–1146 (2007).
Lapointe, C. P. et al. Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation. Proc. Natl Acad. Sci. USA 118, e2017715118 (2021).
Johnson, A. G. et al. RACK1 on and off the ribosome. RNA 25, 881–895 (2019).
Aitken, C. E., Marshall, R. A. & Puglisi, J. D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys. J. 94, 1826–1835 (2008).
Marshall, R. A., Dorywalska, M. & Puglisi, J. D. Irreversible chemical steps control intersubunit dynamics during translation. Proc. Natl Acad. Sci. USA 105, 15364–15369 (2008).
Blanchard, S. C., Kim, H. D., Gonzalez, R. L. Jr., Puglisi, J. D. & Chu, S. tRNA dynamics on the ribosome during translation. Proc. Natl Acad. Sci. USA 101, 12893–12898 (2004).
Grentzmann, G., Ingram, J. A., Kelly, P. J., Gesteland, R. F. & Atkins, J. F. A dual-luciferase reporter system for studying recoding signals. RNA 4, 479–486 (1998).
Ivanov, I. P. et al. Evolutionarily conserved inhibitory uORFs sensitize Hox mRNA translation to start codon selection stringency. Proc. Natl Acad. Sci. USA 119, e2117226119 (2022).
Acknowledgements
We are grateful to members of the C.P.L., T.E.D. and J.D.P. labs for helpful guidance, discussions and feedback. We appreciate helpful feedback from M. Lawson and the Lawson lab (University of California, Los Angeles). We thank P. Sarnow and his lab (Stanford) for sharing cell culture equipment. C.A. is supported by the Howard Hughes Medical Institute Gilliam Fellows Program and a Stanford Bio-X fellowship. This work was funded, in part, by a Chan Zuckerberg Biohub Investigator Award (to J.D.P.), the National Institutes of Health (GM145306 and AG064690 to J.D.P.; GM144678 to C.P.L.), the Intramural Research Program of the National Institutes of Health (to T.E.D.) and the Damon Runyon Cancer Research Foundation (DFS-49-22 to C.P.L.). This research also was supported by the Proteomics and Metabolomics Shared Resource (RRID:SCR_022618) of the Fred Hutch/University of Washington/Seattle Children’s Cancer Consortium (P30 CA015704).
Author information
Authors and Affiliations
Contributions
R.G. and C.A. contributed equally. Conceptualization, R.G., C.A., T.E.D. and C.P.L. Methodology, R.G., C.A., I.P.I., O.B.N., J.D.P., T.E.D. and C.P.L. Resources, R.G., C.A., I.P.I., T.E.D. and C.P.L. Investigation, R.G., C.A., I.P.I., O.B.N., T.E.D. and C.P.L. Visualization, R.G., C.A., T.E.D. and C.P.L. Funding acquisition, J.D.P., T.E.D. and C.P.L. Project administration, T.E.D. and C.P.L. Supervision, J.D.P., T.E.D. and C.P.L. Writing—original draft, C.P.L. Writing—review and editing, R.G., C.A., I.P.I., O.B.N., J.D.P., T.E.D. and C.P.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Katrin Karbstein, Leos Shivaya Valasek and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editors: Sara Osman and Melina Casadio, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 An eIF1 FRET signal.
(a) Image of a gel from SDS-PAGE analysis of purified eIF1 labeled on C94 with Cy5 fluorescent dye. The left and right images are total protein and Cy5 fluorescent scans of the same gel. (b) Image of an acid TBE-Urea gel that examined Met-tRNAi-Cy3 before and after in vitro aminoacylation with methionine. The gel was scanned for Cy3 fluorescence. No molecular weight ladder was used. (c) Schematic of the equilibrium total internal reflection fluorescence microscopy (TIRFm) experiments used to validate the eIF1 FRET signal. Samples were excited with a 532 nM laser. (d) Example fields of view of TIRFm experiments. (e,f) Example fluorescence data of initiation complexes equilibrated on the model mRNA without a start site (No AUG model, panel e) or with a start site (AUG model, panel f). The complexes contain tRNAi-Cy3 (green) to eIF1-Cy5 (red) FRET events. The top plots represent the fluorescence intensities (arbitrary units) of both signals, and the bottom plots represent the calculated FRET efficiency (EFRET) during eIF1-Cy5 binding events. The right panels plot the EFRET distribution observed on the indicated number of eIF1 binding events. The mean EFRET ± standard deviations are indicated. (g) Cumulative probability plot of the eIF1 FRET lifetime observed on the two model mRNAs. The lines represent fits to double-exponential functions, which were used to derive the indicated rates.
Extended Data Fig. 2 eIF1 stably binds and dynamically samples individual complexes.
(a) Schematic of predicted single molecule data for eIF1-Cy5 experiments. Initial appearance of tRNAi-Cy3 (green) to eIF1-Cy5 (red) FRET signifies that a 43S initiation complex loaded onto the tethered mRNA. This initial FRET event is defined as the ‘initial eIF1 binding event’ and the duration of this event defined as the ‘initial eIF1 binding lifetime’. The complex then contains multiple subsequent tRNAi-Cy3 to eIF1-Cy5 FRET events, which are defined as subsequent eIF1 binding events. The dwell time between loss of the previous eIF1 signal and appearance of the next eIF1 signal is defined as the ‘eIF1 rebinding time’. The duration of all subsequent eIF1 binding events is defined as the ‘subsequent eIF1 binding lifetime’. Appearance of eIF5B-Cy3.5 (orange) signal signifies successful entry to the final initiation steps that culminate with joining of the 60S ribosomal subunit. The dwell time from loss of the final eIF1-Cy5 signal to appearance of eIF5B-Cy3.5 signal is defined as the ‘eIF5B binding time’. (b) Cumulative probability plot of the eIF5B binding time measured relative to initial loading of the 43S initiation complex, which was signified by either appearance of tRNAi-Cy3 to eIF1-Cy5 FRET (this study) or initial appearance of 40S-Cy3 signal. The overlapped plots indicate that labeled tRNAi-Cy3 and eIF1-Cy5 function analogously as their unlabeled versions. (c) Plot of the percentage of loaded 43S initiation complexes that contain multiple eIF1 binding events on β-globin mRNA in the indicated conditions. ‘Productive’ indicates complexes that progressed to eIF5B binding, whereas ‘any’ indicates that all complexes were analyzed regardless of whether eIF5B bound. (d) Cumulative probability plots of the indicated parameters at differing Cy5-eIF1 concentrations, observed on any loaded initiation complexes (both successful and unsuccessful). Lines represent fits to exponential functions. (e) Schematic of the real-time single-molecule assay with direct excitation of all fluorophores present using dual 532 nm and 640 nm lasers. This excitation scheme examined whether eIF1-Cy5 fully departed initiation complexes. (f) Example single-molecule data of the direct excitation assay showing termination of eIF1 binding is correlated to loss of FRET. The total number of initiation complexes analyzed is indicated (n = 115) and all 744 eIF1-Cy5 binding events ended with complete loss of eIF1-Cy5 signal. See Supplementary Table 1 for the number of complexes and binding events analyzed in each experiment.
Extended Data Fig. 3 Termination of transient eIF1 binding requires a translation start site and concentration-dependent binding by eIF5.
(a) Cumulative probability plots of the indicated parameters for eIF1-Cy5 binding at the indicated molar ratios of ATP:ADPNP on the 200 nt 5’UTR model RNA. Lines represent fits to exponential functions. (b) Plot of the 43S initiation complex loading efficiency on the mRNA at the indicated ATP:ADPNP ratios (1 mM total concentration) relative to 1 mM ATP. (c) Cumulative probability plot of the 43S initiation complex loading time (from start of data acquisition to appearance of tRNAi-Cy3 fluorescence signal) at the indicated molar ratios of ATP:ADPNP on the 200 nt 5’UTR model RNA. Lines represent fits to exponential functions. (d) Cumulative probability plots of the indicated kinetic parameters in experiments that monitored eIF1-Cy5 on the 50 nt 5’UTR with an AUG start site in the presence of a limiting concentration (5 nM) of eIF1A. Lines represent fits to exponential functions. The rightmost plot quantifies the percentage of loaded 43S initiation complexes that ultimately progressed to eIF5B binding, which was corrected for the eIF5B labeling efficiency. (e,g) Representative single-molecule fluorescence data that monitored eIF1-Cy5 either in the presence of TC-GDPNP (panel e) or in the presence of a limiting concentration of eIF5 (10 nM) (panel g). Both experiments used the β-globin mRNA. (f) Cumulative probability plots of the indicated kinetic parameters in experiments that monitored eIF1-Cy5 on the on β-globin mRNA in the presence of different concentrations of eIF5. Lines represent fits to exponential functions. See Supplementary Table 1 for the number of complexes and binding events analyzed in each experiment.
Extended Data Fig. 4 An eIF5 FRET signal.
(a) Image of a gel from SDS-PAGE analysis of purified eIF5 labeled on its N-terminal ybbR tag with Cy5 (or Cy5.5) fluorescent dye. The left and right images are total protein and Cy5 fluorescent scans of the same gel. Identical results are obtained with either Cy5 or Cy5.5. (b) Schematic of the equilibrium total internal reflection fluorescence microscopy (TIRFm) experiments. Samples were excited with a 532 nM laser. (c) Example field of view of TIRFm experiments. (d) Example fluorescence data of initiation complexes equilibrated on the model mRNA with an AUG start site. The complexes contain tRNAi-Cy3 (green) to eIF5-Cy5 (purple) FRET events. The top plot represents the fluorescence intensities (arbitrary units) of both signals, and the bottom plot represents the calculated FRET efficiency (EFRET). The right panel plots the EFRET distribution observed during the indicated number of eIF5 binding events. The mean EFRET ± standard deviations are indicated. (e) Cumulative probability plot of the eIF5 FRET lifetime observed on the AUG model mRNA. The line represents a fit to a double-exponential function, which was used to derive the indicated rates.
Extended Data Fig. 5 eIF5-Cy5.5 functions analogously to its unlabeled version.
(a) Example single-molecule fluorescence data from an experiment that monitored loading of the 43S initiation complex as signaled by 40S-Cy3 (green) in the presence of 40 nM eIF5B-Cy3.5 (orange), and 60S-Cy5 (red). In this experiment, unlabeled eIF1 and eIF5 were present at 290 nM each. (b-d) Cumulative probability plot of the eIF5B binding time, 60S subunit joining time, and the lifetime of eIF5B on the 80S initiation complex. The eIF5B binding time was measured relative to initial loading of the 43S initiation complex, which was signified by either appearance of tRNAi-Cy3 signal (this study) or initial appearance of 40S-Cy3 signal22. The overlapped plots indicate that labeled tRNAi-Cy3 and eIF5-Cy5.5 function analogously as their unlabeled versions.
Extended Data Fig. 6 eIF5 transiently binds initiation complexes.
(a) Schematic of predicted single-molecule data that monitor eIF5-Cy5.5. Initial appearance of tRNAi-Cy3 (green) fluorescence indicates loading of the 43S initiation complex onto the mRNA. Either direct appearance of eIF5-Cy5.5 (purple) fluorescence or appearance of tRNAi-to-eIF5 FRET indicates the presence of eIF5 on initiation complexes. Appearance of eIF5B-Cy3.5 (orange) indicates binding of eIF5B. Appearance of 60S-Cy5 (red) fluorescence indicates the 60S subunit joined to form the 80S ribosome; the relative proximity of tRNAi-Cy3 and 60S-Cy5 labeling sites in the 80S initiation complex yields low FRET. The kinetic parameters were defined as follows. ‘eIF5 first binding time’ as the time elapsed from initial appearance of the tRNAi-Cy3 signal to the first appearance of eIF5-Cy5.5 signal. ‘eIF5 first lifetime’ as the duration of the first eIF5-Cy5.5 binding event. ‘eIF5 rebinding time’ as the time elapsed from departure of the previous eIF5 protein until the start of the next binding event. ‘eIF5 subsequent lifetimes’ as the duration of the subsequent eIF5 binding events. ‘eIF5 final binding time’ as the time elapsed from appearance of the tRNAi-Cy3 signal until appearance of the final eIF5-Cy5.5 signal prior to eIF5B binding. ‘eIF5 final lifetime’ was the duration of the final eIF5 binding event. Since most complexes contained a single eIF5 binding event, the ‘first’ and ‘final’ eIF5 binding events were identical on most complexes. ‘eIF5B binding time’ was defined as the time elapsed from disappearance of the final eIF5 signal until appearance of the eIF5B-Cy3.5 signal. ‘60S joining time’ was defined as the time elapsed from appearance of eIF5B-Cy3.5 signal until appearance of 60S-Cy5 signal. ‘eIF5B-80S lifetime’ was defined as the duration of the eIF5B-Cy3.5 signal on the 80S initiation complex. (b) Cumulative probability plots of the indicated kinetic parameters on β-globin mRNA at the indicated concentrations of eIF5-Cy5.5. (c) Example single-molecule data of an initiation complex that was bound multiple times by eIF5-Cy5.5. (d) Plot of the percent of initiation complexes that contained either a single or multiple eIF5-Cy5.5 binding events at the indicated eIF5-Cy5.5 concentrations. (e) Plot of the percent of initiation complexes where eIF5B bound either during (overlapped), within 100 ms of ( < 0.1 s), or after 100 ms ( > 0.1 s) the final eIF5 binding event at the indicated concentrations of eIF5-Cy5.5. In all experiments, unlabeled eIF1 was present at a final concentration of 290 nM and eIF5-Cy5.5 was present at the indicated final concentrations (by Cy5.5 dye). See Supplementary Table 3 for the number of complexes and binding events analyzed in each experiment.
Extended Data Fig. 7 eIF5 competes with eIF1 to bind initiation complexes.
(a) Plot of the percentage of initiation complexes bound by eIF5 in the presence of 290 nM or 10 nM eIF1 on the AUG model RNA. (b) Cumulative probability plot of the eIF5 first binding time on initiation complexes in the presence of 290 nM or 10 nM eIF1 on the AUG model RNA. (c) Schematic of the single-molecule translation initiation assay to monitor eIF1-Cy5 and eIF5-Cy5.5 binding simultaneously using the AUG model RNA. In this experiment, only the 532 nm excitation laser was used. This experimental scheme directly detected loading of the 43S initiation complex (via tRNAi-Cy3, green) and eIF5B-Cy3.5 (orange), as the conjugated dyes are directly excited by the 532 nm laser. By contrast, eIF1-Cy5 (red) and eIF5-Cy5.5 (purple) are not excited directly by the 532 nm laser and thus their binding is detected solely via FRET with the tRNAi-Cy3 donor. (d) Representative single-molecule data of initiation complexes that progressed to eIF5B binding using the experimental scheme outlined in panel c. In total, 77 initiation complexes were analyzed, and all eIF1 and eIF5 binding events were mutually exclusive. Furthermore, eIF5 was the final binding event prior to eIF5B binding on 95% of the complexes. We suspect that the remaining 5% where eIF1 was last were likely followed by binding of an unlabeled eIF5 protein immediately prior to eIF5B binding. (e) Schematic of an alternative single-molecule translation initiation assay to monitor eIF1-Cy5 and eIF5-Cy5.5 binding simultaneously using the AUG model RNA. In this experiment, 532 nm and 640 nm excitation lasers were used simultaneously. This experimental scheme directly detected binding of all fluorescently-labeled components in the assays, independent of FRET. (f) Representative single-molecule data of initiation complexes that progressed to eIF5B binding using the experimental scheme outlined in panel e. As in panels c and d, eIF1 and eIF5 binding was mutually exclusive on initiation complexes that progressed to eIF5B binding. (g) On about 5% of complexes, we observed eIF1 and eIF5 binding events that overlapped; however, these complexes never progressed to eIF5B binding (that is, were unsuccessful) and very likely represent aberrant complexes or non-specific binding events.
Extended Data Fig. 8 eIF1 binding kinetics depend on the identity of the translation start site.
(a) Cumulative probability plots of the indicated eIF1 kinetic parameters on eIF5B-bound initiation complexes (successful) on the model AUG mRNAs in ideal (ACCAUGGA) or poor (CCCAUGCA) Kozak context. Lines represent fits to exponential functions. (b) Cumulative probability plots of the indicated eIF1 parameters observed on any loaded initiation complexes (both unsuccessful and successful) on the indicated model RNAs. Lines represent fits to exponential functions. (c) Plot of the population-weighted mean elapsed times for the indicated eIF1 parameters on the indicated mRNAs. All values plotted here were derived from events that occurred on any loaded initiation complex (both unsuccessful and successful). (d,e) Cumulative probability plots of the indicated kinetic parameters on eIF5B-bound initiation complexes on the indicated model mRNAs in the presence of 290 nM (panel d) or 5 nM (panel e) unlabeled eIF5. Lines represent fits to exponential functions. (f) Plot of the population-weighted mean eIF1 binding time on the indicated model RNAs in the presence of molar excess eIF5 (290 nM) or a limiting eIF5 concentration of 5 nM. (g) Plot of the percent of initiation complexes that classified into the three broad categories of eIF1-Cy5 binding outlined in Fig. 4b on β-globin mRNA. ‘Indeterminant’ captures complexes that were too ambiguous to classify. In all experiments, eIF1-Cy5 was present at a final concentration of 40 nM (by Cy5) and unlabeled eIF5 was present at either 290 or, when indicated, at 5 nM. See Supplementary Table 1 for the number of complexes and binding events analyzed in each experiment.
Extended Data Fig. 9 eIF5 binding kinetics depend on the identity of the translation start site.
(a) Cumulative probability plots of the indicated eIF5 kinetic parameters on eIF5B-bound initiation complexes on the model AUG RNAs in ideal (ACCAUGGA) or poor (CCCAUGCA) Kozak context. Here, the eIF5B binding time was measured relative to loss of signal for the final eIF5-Cy5.5 binding event. The negative times represent complexes where eIF5B bound before eIF5 departed. Lines represent fits to exponential functions. (b) Cumulative probability plots of the indicated eIF5 kinetic parameters on eIF5B-bound initiation complexes on the model RNAs with various non-AUG start sites in ideal Kozak context. Here, the eIF5B binding time was measured relative to loss of signal for the final eIF5-Cy5.5 binding event. The negative times represent complexes where eIF5B bound before eIF5 departed. Lines represent fits to exponential functions. Lines represent fits to exponential functions. (c) Cumulative probability plots of the indicated eIF5 kinetic parameters on eIF5B-bound initiation complexes on the AUG or GUG model RNAs in the presence of a limiting concentration of eIF1 (10 nM). Here, the eIF5B binding time was quantified relative to loading of the 43S initiation complex to demonstrate initiation proceeded at similar rates on AUG and GUG start sites when the concentration of eIF1 was limiting. (d,e) Cumulative probability plots of the eIF5B binding time measured relative to loading of the 43S initiation complex when eIF1-Cy5 was monitored in the presence of excess eIF5 (panel d) or eIF5-Cy5.5 was monitored in the presence of excess eIF1 (panel e). (f) A replot of cumulative probability data depicted in panels d and e to facilitate cross-comparisons of the data. See Supplementary Table 3 for the number of complexes and binding events analyzed in each experiment.
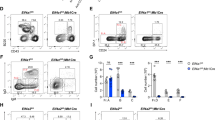
Extended Data Fig. 10 Perturbing eIF1 and eIF5 expression in human cells.
Immunoblots measuring eIF1 and eIF5 levels in U2OS cells transfected with plasmids overexpressing eIF1, eIF5, or a control empty vector (panels a-c); or eIF1 shRNA, eIF5 shRNA, or a scrambled control shRNA (panels d-f). The blots depict three replicates for each sample, each run on a single lane of the gel. Bands for eIF1, eIF5, and β-actin were quantified using ImageJ and then averaged (mean). eIF1/β-actin and eIF5/β-actin ratios were calculated based on the quantifications and plotted as normalized values to the control samples. All error bars denote SD. * denotes p < 0.05 from student’s two-tailed t test for n = 3 replicates relative to the control sample. Precise p-values are (from left to right): panel b, 0.0047; panel c, 0.025; panel f, 0.039 and 0.042.
Supplementary information
Supplementary Table 1
Supplementary Table 1. All rates, rate constants and the number of complexes and binding events analyzed in each real-time single molecule experiment using eIF1–Cy5. Supplementary Table 2. All proteins identified in the final eIF3 protein sample used in the single-molecule assays. eIF1 and eIF5 were not detected. Supplementary Table 3. All rates, rate constants and the number of complexes and binding events analyzed in each real-time single molecule experiment using eIF5–Cy5.5.
Source data
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Extended Data Fig. 10
Source data.
Source Data
Full gel and western blot images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grosely, R., Alvarado, C., Ivanov, I.P. et al. eIF1 and eIF5 dynamically control translation start site fidelity. Nat Struct Mol Biol (2025). https://doi.org/10.1038/s41594-025-01629-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-025-01629-y
This article is cited by
-
Two dynamically competing factors determine the translation start site
Nature Structural & Molecular Biology (2025)