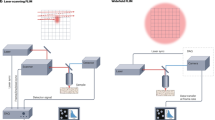
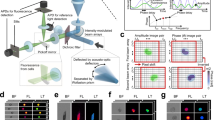
Abstract
Measuring forces within living cells remains a technical challenge. In this Tutorial, we cover the development of hydrophobic mechanosensing fluorescent probes called Flippers, whose fluorescence lifetime depends on lipid packing. Flipper probes can therefore be used as reporters for membrane tension via the measurement of changes in their fluorescence lifetime. We describe the technical optimization of the probe for imaging and provide working examples for their characterizations in a variety of biological and in vitro systems. We further provide a guideline to measure biophysical parameters of cellular membranes by fluorescence lifetime imaging microscopy using Flipper probes, providing evidence that flippers can report long range forces in cells, tissues and organs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Roca-Cusachs, P., Conte, V. & Trepat, X. Quantifying forces in cell biology. Nat. Cell Biol. 19, 742–751 (2017).
Abella, M., Andruck, L., Malengo, G. & Skruzny, M. Actin-generated force applied during endocytosis measured by Sla2-based FRET tension sensors. Dev. Cell 56, 2419–2426.e4 (2021).
Déjardin, T. et al. Nesprins are mechanotransducers that discriminate epithelial–mesenchymal transition programs. J. Cell Biol. 219, e201908036 (2020).
Li, W. et al. A membrane-bound biosensor visualizes shear stress-induced inhomogeneous alteration of cell membrane tension. iScience 7, 180–190 (2018).
Páez-Pérez, M., López-Duarte, I., Vyšniauskas, A., Brooks, N. J. & Kuimova, M. K. Imaging non-classical mechanical responses of lipid membranes using molecular rotors. Chem. Sci. 12, 2604–2613 (2021).
Assies, L. et al. Flipper probes for the community. Chim. Int. J. Chem. 75, 1004–1011 (2021).
Soleimanpour, S. et al. Headgroup engineering in mechanosensitive membrane probes. Chem. Commun. 52, 14450–14453 (2016).
Colom, A. et al. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125 (2018).
Mercier, V. et al. Endosomal membrane tension regulates ESCRT-III-dependent intra-lumenal vesicle formation. Nat. Cell Biol. 22, 947–959 (2020).
Roffay, C. et al. Passive coupling of membrane tension and cell volume during active response of cells to osmosis. Proc. Natl Acad. Sci. USA 118, e2103228118 (2021).
Ni, Q. et al. Cytoskeletal activation of NHE1 regulates cell volume and DNA methylation. Preprint at bioRxiv https://doi.org/10.1101/2023.08.31.555808 (2023).
Michels, L. et al. Complete microviscosity maps of living plant cells and tissues with a toolbox of targeting mechanoprobes. Proc. Natl Acad. Sci. USA 117, 18110–18118 (2020).
Coomer, C. A. et al. Single-cell glycolytic activity regulates membrane tension and HIV-1 fusion. PLoS Pathog. 16, e1008359 (2020).
Jiménez-Rojo, N. et al. Conserved functions of ether lipids and sphingolipids in the early secretory pathway. Curr. Biol. 30, 3775–3787.e7 (2020).
Mylvaganam, S. et al. The spectrin cytoskeleton integrates endothelial mechanoresponses. Nat. Cell Biol. 24, 1226–1238 (2022).
Lachuer, H., Le, L., Lévêque-Fort, S., Goud, B. & Schauer, K. Spatial organization of lysosomal exocytosis relies on membrane tension gradients. Proc. Natl Acad. Sci. 120, e2207425120 (2023).
Lachowski, D. et al. Substrate stiffness-driven membrane tension modulates vesicular trafficking via caveolin-1. ACS Nano 16, 4322–4337 (2022).
Riggi, M. et al. Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2. Nat. Cell Biol. 20, 1043–1051 (2018).
Riggi, M., Kusmider, B. & Loewith, R. The flipside of the TOR coin—TORC2 and plasma membrane homeostasis at a glance. J. Cell Sci. 133, jcs242040 (2020).
Wang, S. et al. Adipocyte Piezo1 mediates obesogenic adipogenesis through the FGF1/FGFR1 signaling pathway in mice. Nat. Commun. 11, 2303 (2020).
Nava, M. M. et al. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181, 800–817.e22 (2020).
Schneider, A. F. L., Kithil, M., Cardoso, M. C., Lehmann, M. & Hackenberger, C. P. R. Cellular uptake of large biomolecules enabled by cell-surface-reactive cell-penetrating peptide additives. Nat. Chem. 13, 530–539 (2021).
Hetmanski, J. H. R. et al. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell 51, 460–475.e10 (2019).
Yavitt, F. M. et al. In situ modulation of intestinal organoid epithelial curvature through photoinduced viscoelasticity directs crypt morphogenesis. Sci. Adv. 9, eadd5668 (2023).
Goujon, A. et al. Mechanosensitive fluorescent probes to image membrane tension in mitochondria, endoplasmic reticulum, and lysosomes. J. Am. Chem. Soc. 141, 3380–3384 (2019).
Dal Molin, M. et al. Fluorescent flippers for mechanosensitive membrane probes. J. Am. Chem. Soc. 137, 568–571 (2015).
García-Sáez, A. J., Chiantia, S. & Schwille, P. Effect of line tension on the lateral organization of lipid membranes. J. Biol. Chem. 282, 33537–33544 (2007).
Hamada, T., Kishimoto, Y., Nagasaki, T. & Takagi, M. Lateral phase separation in tense membranes. Soft Matter 7, 9061–9068 (2011).
Shimokawa, N. & Hamada, T. Physical concept to explain the regulation of lipid membrane phase separation under isothermal conditions. Life 13, 1105 (2023).
García-Calvo, J. et al. HydroFlipper membrane tension probes: imaging membrane hydration and mechanical compression simultaneously in living cells. Chem. Sci. 13, 2086–2093 (2022).
Licari, G., Strakova, K., Matile, S. & Tajkhorshid, E. Twisting and tilting of a mechanosensitive molecular probe detects order in membranes. Chem. Sci. 11, 5637–5649 (2020).
Harris, F. M., Best, K. B. & Bell, J. D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta 1565, 123–128 (2002).
Golfetto, O., Hinde, E. & Gratton, E. Laurdan fluorescence lifetime discriminates cholesterol content from changes in fluidity in living cell membranes. Biophys. J. 104, 1238–1247 (2013).
Ragaller, F. et al. Quantifying fluorescence lifetime responsiveness of environment sensitive probes for membrane fluidity measurements. J. Phys. Chem. B. 128, 2154–2167 (2024).
Piazzolla, F. et al. Fluorescent membrane tension probes for early endosomes. Angew. Chem. Int. Ed. 133, 12366–12371 (2021).
García-Calvo, J. et al. Fluorescent membrane tension probes for super-resolution microscopy: combining mechanosensitive cascade switching with dynamic-covalent ketone chemistry. J. Am. Chem. Soc. 142, 12034–12038 (2020).
López-Andarias, J. et al. Photocleavable fluorescent membrane tension probes: fast release with spatiotemporal control in inner leaflets of plasma membrane, nuclear envelope, and secretory pathway. Angew. Chem. Int. Ed. 61, e202113163 (2022).
Gao, D. et al. FLIMJ: an open-source ImageJ toolkit for fluorescence lifetime image data analysis. PLoS ONE 15, e0238327 (2020).
Warren, S. C. et al. Rapid global fitting of large fluorescence lifetime imaging microscopy datasets. PLoS ONE 8, e70687 (2013).
Köllner, M. & Wolfrum, J. How many photons are necessary for fluorescence-lifetime measurements? Chem. Phys. Lett. 200, 199–204 (1992).
Parasassi, T., Gratton, E., Yu, W. M., Wilson, P. & Levi, M. Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys. J. 72, 2413–2429 (1997).
Zlotek-Zlotkiewicz, E., Monnier, S., Cappello, G., Le Berre, M. & Piel, M. Optical volume and mass measurements show that mammalian cells swell during mitosis. J. Cell Biol. 211, 765–774 (2015).
Fischer-Friedrich, E., Hyman, A. A., Jülicher, F., Müller, D. J. & Helenius, J. Quantification of surface tension and internal pressure generated by single mitotic cells. Sci. Rep. 4, 1–8 (2014).
Stewart, M. P. et al. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature 469, 226–230 (2011).
Storck, E. M., Özbalci, C. & Eggert, U. S. Lipid cell biology: a focus on lipids in cell division. Annu. Rev. Biochem. 87, 839–869 (2018).
Atilla-Gokcumen, G. E. et al. Dividing cells regulate their lipid composition and localization. Cell 156, 428–439 (2014).
Andrade, V. et al. Caveolae promote successful abscission by controlling intercellular bridge tension during cytokinesis. Sci. Adv. 8, eabm5095 (2022).
Royer, C. et al. ASPP2 maintains the integrity of mechanically stressed pseudostratified epithelia during morphogenesis. Nat. Commun. 13, 941 (2022).
Acknowledgements
We thank S. Srinivas and C. Royer (University of Oxford) for isolating and staining the mouse embryos. F.S. is grateful for the support from the EMBO (ALTF 849-2020) and HFSP (LT000404/2021-L) long-term postdoctoral fellowships. F.S. thanks S. Fraser (University of Southern California) for the support and supervision and acknowledges the Translational Imaging Center (University of Southern California) for access to instrumentation and expertise. A.C. also acknowledges funding from MCIU, PID2019-111096GA-I00; MCIU/AEI/FEDER MINECOG19/P66, RYC2018-024686-I and the Basque Government T1270-19. V.D. thanks P.-F. Lenne (IBDM Marseille) for supervision and resources. V.D. acknowledges support by an HFSP long-term postdoctoral fellowship (HFSP LT0058/2022-L) and the France–BioImaging infrastructure supported by the French National Research Agency (ANR–10–INBS- 04-01, Investments for the future). V.D. thanks F. Schnorrer (IBDM Marseille) for access to the FLIM system. S.M. thank the University of Geneva, the National Centre of Competence in Research Chemical Biology (51NF40-185898), the National Centre of Competence in Research Molecular Systems Engineering (51NF40-182895) and the Swiss NSF (Excellence Grant 200020 204175; SNSF-ERC Advanced Grant TIMEUP, TMAG-2_209190) for financial support. A.R. acknowledges funding from the Swiss National Fund for Research Grants nos. 310030_200793 and no. CRSII5_189996 and the European Research Council Synergy Grant no. 951324 R2-TENSION.
Author information
Authors and Affiliations
Contributions
The project was designed by C.R., A.R. and V.M. C.R. and V.M. carried out most of the experiments and analyses. J.M.G.-A. performed the experiments on bacteria, P.C. performed some experiments on MDCK, C.T. performed experiments on alginate tubes, F.S. performed the biphoton experiments, I.D.M. performed experiments with alginate capsules, A.C. performed experiments on Arabidopsis, K.B. and V.D. performed the experiments on Xenopus and J.L.-A. and S.M. designed and synthesized the Flippers molecules. C.R., V.M. and A.R. wrote the paper, with corrections from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 FLIM of Flipper-TR using two-photon microscopy.
(a) Absorption and excitation spectrum of the Flipper-TR. (b) Two-photon excitation spectra of Flipper-TR, acquired in 48h gastruloids. Five gastruloids were imaged in total at each wavelength. The acquired spectra were normalized to the maximum intensity value. Dots represented the average value, scattered lines the minimum/ maximum values of the 5 recorded spectra. (c) and (d): Lifetime of Flipper-TR in GUVs of different compositions DOPC (c) and 1:4 DOPC:Cholesterol (d) at different laser repetition rates and under two-photon excitation. Every dot represents fitted lifetime for 1 GUV, error bars are standard deviation and line is mean. (e) Fluorescence lifetime images of GUVs from (c, d) under typical excitation conditions (20 MHz, 488 nm, top panel) and under two-photon excitation (80 MHz, 900 nm, bottom panel). Longer lifetime component is shown (shorter lifetime component fixed). Image size is 36.4 × 36.4 µm2.
Extended Data Fig. 2 Effect of linearly polarized excitation light on Flipper-TR lifetime measurements in GPMVs and GUVs.
(a) Intensity and (b) Lifetime images of cell-derived vesicles. Photoselection of the fixed excitation dipole of the Flipper-TR probe (using a linearly polarized excitation laser) causes bright rings with higher intensity. Graphs show (c) Lifetime and (d) intensity analysis of bright vs dark regions. Every dot represents the lifetime fit across one vesicle. The line represents the mean and error bars are standard deviation. (e) Intensity and (f) lifetime and image of artificial vesicle composed of DOPC and Cholesterol (4:1). Image size is 36.4*36.4 µm2. The graphs represent (g) lifetime and (h) intensity analysis of bright vs dark regions. Every dot represents the lifetime fit across one vesicle. The line represents the mean and error bars are standard deviation.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roffay, C., García-Arcos, J.M., Chapuis, P. et al. Tutorial: fluorescence lifetime microscopy of membrane mechanosensitive Flipper probes. Nat Protoc 19, 3457–3469 (2024). https://doi.org/10.1038/s41596-024-01027-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01027-6