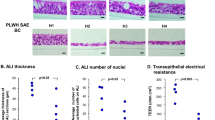
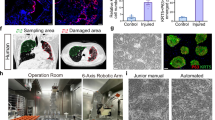
Abstract
Durable and functional regeneration of the airway epithelium in vivo with transplanted stem cells has the potential to reconstitute healthy tissue in diseased airways, such as in cystic fibrosis or primary ciliary dyskinesia. Here, we present detailed protocols for the preparation and culture expansion of murine primary and induced pluripotent stem cell-derived airway basal stem cells (iBCs) and methods for their intra-airway transplantation into polidocanol-conditioned murine recipients to achieve durable in vivo airway regeneration. Reconstitution of the airway tissue resident epithelial stem cell compartment of immunocompetent mice with syngeneic donor cells leverages the extensive self-renewal and multipotent differentiation properties of basal stem cells (BCs) to durably generate a broad diversity of mature airway epithelial lineages in vivo. Engrafted donor-derived cells re-establish planar cell polarity as well as functional ciliary transport. By using this same approach, human primary BCs or iBCs transplanted into NOD-SCID gamma recipient mice similarly display engraftment and multilineage airway epithelial differentiation in vivo. The time to generate mouse or human iBCs takes ~60 d, which can be reduced to ~20 d if previously differentiated cells are thawed from cryopreserved iBC archives. The tracheal conditioning regimen and cell transplantation procedure is completed in 1 d. A competent graduate student or postdoctoral trainee should be able to perform the procedures listed in this protocol.
Key points
-
This protocol outlines methods for the culture of primary or pluripotent stem cell-derived basal cells in vitro, the engraftment of these cells in vivo into immunocompetent mice and similarly the xenotransplantion of human primary or pluripotent stem cell-derived basal cells into immunocompromised mouse recipients.
-
The approach enables reconstitution of the in vivo airway stem cell compartment and, importantly, uses syngeneic cell transplantation into mice with normal immune function rather than into immunodeficient recipients, simulating future autologous cell-based therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
This article includes publicly available transcriptomic datasets, and the bioinformatics files have been deposited at the Gene Expression Ombinus (accession number: GSE232545). There are no restrictions on data sharing, and any raw data, cell lines or protocol downloads are available through the corresponding author by email request or through our website portal at www.kottonlab.com.
Change history
31 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41596-025-01180-6
References
Vaughan, A. E. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015).
Nichane, M. et al. Isolation and 3D expansion of multipotent Sox9+ mouse lung progenitors. Nat. Methods 14, 1205–1212 (2017).
Kathiriya, J. J., Brumwell, A. N., Jackson, J. R., Tang, X. & Chapman, H. A. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell 26, 346–358.e4 (2020).
Liao, C.-C., Chiu, C.-J., Yang, Y.-H. & Chiang, B.-L. Neonatal lung-derived SSEA-1+ cells exhibited distinct stem/progenitor characteristics and organoid developmental potential. iScience 25, 104262 (2022).
Louie, S. M. et al. Progenitor potential of lung epithelial organoid cells in a transplantation model. Cell Rep. 39, 110662 (2022).
Ghosh, M., Ahmad, S., White, C. W. & Reynolds, S. D. Transplantation of airway epithelial stem/progenitor cells: a future for cell-based therapy. Am. J. Resp. Cell Mol. Biol. 56, 1–10 (2016).
Fujimura, T. et al. Identifying a lung stem cell subpopulation by combining single-cell morphometrics, organoid culture, and transcriptomics. Stem Cells 41, 809–820 (2023).
Miller, A. J. et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119 (2018).
Ma, L. et al. Airway stem cell reconstitution by the transplantation of primary or pluripotent stem cell-derived basal cells. Cell Stem Cell 30, 1199–1216.e7 (2023).
Serra, M. et al. Pluripotent stem cell differentiation reveals distinct developmental pathways regulating lung- versus thyroid-lineage specification. Development 144, 3879–3893 (2017).
McCauley, K. B. et al. Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem Cell Rep. 10, 1579–1595 (2018).
Ikonomou, L. et al. The in vivo genetic program of murine primordial lung epithelial progenitors. Nat. Commun. 11, 635 (2020).
Kubo, A. et al. Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651–1662 (2004).
Green, M. D. et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 29, 267–272 (2011).
Longmire, T. A. et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398–411 (2012).
McCauley, K. B. et al. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 20, 844–857.e6 (2017).
Mou, H. et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 19, 217–231 (2016).
Hawkins, F. J. et al. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell 28, 79–95.e8 (2021).
Morrison, S. J., Uchida, N. & Weissman, I. L. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11, 35–71 (1995).
Borthwick, D. W., Shahbazian, M., Krantz, Q. T., Dorin, J. R. & Randell, S. H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Resp. Cell Mol. Biol. 24, 662–670 (2012).
Fulcher, M. L. & Randell, S. H. in Epithelial Cell Culture Protocols 2nd edn (eds Fulcher, M. L. & Randell, S. H.) 109–121 (Humana Press, 2012).
Roy, M. G. et al. Muc5b is required for airway defence. Nature 505, 412–416 (2014).
Ostrowski, L. E. et al. Conditional deletion of Dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am. J. Resp. Cell Mol. Biol. 43, 55–63 (2010).
Snouwaert, J. N. et al. An animal model for cystic fibrosis made by gene targeting. Science 257, 1083–1088 (1992).
Bilodeau, M., Shojaie, S., Ackerley, C., Post, M. & Rossant, J. Identification of a proximal progenitor population from murine fetal lungs with clonogenic and multilineage differentiation potential. Stem Cell Rep. 3, 634–649 (2014).
Kurmann, A. A. et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17, 527–542 (2015).
Herriges, M. J. et al. Durable alveolar engraftment of PSC-derived lung epithelial cells into immunocompetent mice. Cell Stem Cell 30, 1217–1234.e7 (2023).
Plasschaert, L. W. et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381 (2018).
Suzuki, S. et al. Differentiation of human pluripotent stem cells into functional airway basal stem cells. STAR Protoc. 2, 100683 (2021).
Gentzsch, M. et al. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am. J. Resp. Cell Mol. Biol. 56, 568–574 (2016).
Zhang, C. et al. Long-term in vitro expansion of epithelial stem cells enabled by pharmacological inhibition of PAK1-ROCK-Myosin II and TGF-β signaling. Cell Rep. 25, 598–610.e5 (2018).
Skarnes, W. C., Pellegrino, E. & McDonough, J. A. Improving homology-directed repair efficiency in human stem cells. Methods 164, 18–28 (2019).
Longmire, T., Ikonomou, L. & Kotton, D. Mouse ESC differentiation to Nkx2.1+ lung and thyroid progenitors. Bio Protoc. 2, e295 (2012).
Dame, K. et al. Thyroid progenitors are robustly derived from embryonic stem cells through transient, developmental stage-specific overexpression of Nkx2-1. Stem Cell Rep. 8, 216–225 (2017).
Wilson, A. A. et al. Amelioration of emphysema in mice through lentiviral transduction of long-lived pulmonary alveolar macrophages. J. Clin. Invest. 120, 379–389 (2010).
Acknowledgements
We thank B. R. Tilton and the entire BUSM Flow Cytometry Core and Y. Alekseyev and the entire BUSM Microarray and Sequencing Resource Core for their technical assistance. We thank the staff of the Marsico Lung Institute Tissue Procurement and Cell Culture Core for primary human cells and media. We thank the entire Kotton and Hawkins Laboratories and the Center for Regenerative Medicine for their support and suggestions throughout the course of this research. We are indebted to G. Miller and M. James for overall laboratory support as well as for reprogramming and characterization of iPSC lines. We are also grateful to L. Ikonomou for his insight and input throughout the project. This work was supported by NHLBI Progenitor Cell Translational Consortium (PCTC) Jumpstart Award to L.M.; Boston University Kilachand Multicellular Design Program Accelerator Grants to L.M., M.J.H. and D.N.K.; NIH grants U01HL134745, U01HL134766, U01HL148692, R01HL095993 and P01HL170952 to D.N.K.; NIH grant R01HL139799 and Cystic Fibrosis Foundation Grant HAWKIN20XX2 to F.J.H.; and NIH grants R01HL124392 and R21HD094012 to X.V. Human cell biobanking and sharing were supported by NHLBI grant NO1: 75N92020C00005 to D.N.K. Schematics in figures were created with Biorender.com.
Author information
Authors and Affiliations
Contributions
L.M., F.J.H. and D.N.K. conceptualized the project. L.M. and M.J.H. developed methods for mouse donor cell preparation. J.A.L.S. developed methods for human donor cell preparation. L.M. and B.R.T. developed methods for donor cell transplantation. L.M. and A.T.-L. performed recipient animal analysis in the manuscript. P.S.B., F.W. and L.M. performed bioinformatic analysis. L.M. and D.N.K. wrote the manuscript. X.V., F.J.H. and D.N.K. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Ma, L. et al. Cell Stem Cell 30, 1199–1216.e7 (2023): https://doi.org/10.1016/j.stem.2023.07.014
Herriges, M. J. et al. Cell Stem Cell 30, 1217–1234.e7 (2023): https://doi.org/10.1016/j.stem.2023.07.016
Hawkins, F. J. et al. Cell Stem Cell 28, 79–95.e8 (2021): https://doi.org/10.1016/j.stem.2020.09.017
McCauley, K. B. et al. Cell Stem Cell 20, 844–857.e6 (2017): https://doi.org/10.1016/j.stem.2017.03.001
Serra, M. et al. Development 144, 3879–3893 (2017): https://doi.org/10.1242/dev.150193
Supplementary information
Supplementary Information
Figure S1. Donor-derived cells do not alter the overall recipient airway structure or cause excessive immune influx. H&E and immunofluorescence confocal microscopy (GFP, CD45 and α-SMA) staining of an old (≥1 y after transplantation) recipient and a control animal that never had airway injury or donor cell transplantation. Both coronal and longitudinal samples are shown. Scale bar = 200 μm.
Supplementary Video 1
Video S1. Mucociliary clearance assay in iBC recipient.
Supplementary Video 2
Video S2. Mucociliary clearance bead assay control.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, L., Thapa, B.R., Le Suer, J.A. et al. Life-long functional regeneration of in vivo airway epithelium by the engraftment of airway basal stem cells. Nat Protoc 20, 810–842 (2025). https://doi.org/10.1038/s41596-024-01067-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01067-y