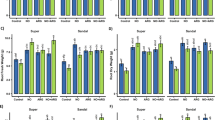
Abstract
Salt stress is an adverse environmental condition that harms plant growth and development. The development of salt stress probes is critical for tracking the growth dynamics of plants, molecular breeding or screening of growth regulators. The sodium chloride (NaCl)-responsive fluorescent probe Aza-CyBz is designed based on the tenet that NaCl induces formation of ordered aggregates, and the sensitive fluorescence response can enable the visualization of plant salt stress in root tip tissues and live plants. Herein, we describe a detailed three-step route for synthesis of Aza-CyBz and applications to monitoring salt stress in Arabidopsis thaliana. The procedures for operating fluorescence imaging under various stresses are also listed to eliminate interference from the oxidative mechanism of salt stress. Compared with conventional invasive approaches such as inductively coupled plasma emission spectrometry and flame photometer, our protocol can real-time monitor salt stress experienced by plants, which demands simple pretreatment procedure and staining technique. Due to near infrared fluorescence, this method provides direct visual observation of salt stress at both tissue and live plant levels, which is superior to conventional noninvasive approaches. The preparation of probe Aza-CyBz takes ~2 d, and the imaging experiments for assessing salt stress experienced by plants, including the preparation of stressed plant samples takes ~9–11 d for root tip tissues and ~23 d for live plants. Notably, acquisition and analysis visual images of salt stress in plants can be completed within 2 h and they require only a basic knowledge of spectroscopy and chemistry.
Key points
-
It is useful to measure NaCl uptake in plants noninvasively, e.g., to discover variants with better salt tolerance. This protocol describes how to prepare and use a NaCl-responsive fluorescent probe Aza-CyBz to image Arabidopsis root tips or whole plants.
-
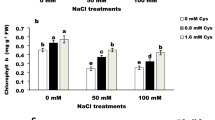
The probe itself has an interesting mechanism: the presence of NaCl results in its aggregation and a reduction in fluorescence. The near infrared fluorescence could avoid background fluorescence interference from chlorophyll.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All supporting data are included in the article, its supplementary information, the supporting research paper and figshare. When the plants were photographed, each photograph had three plants. The images were cropped to show individual plants. In some cases, the cropped photographs still contained parts of other plants and these were hidden using black background. All unedited versions of main and supplementary figures can be found in figshare (https://doi.org/10.6084/m9.figshare.28985144).
References
Farooq, M. et al. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Biochem. 118, 199–217 (2017).
Ma, L., Liu, X., Lv, W. & Yang, Y. Molecular mechanisms of plant responses to salt stress. Front. Plant Sci. 13, 934877 (2022).
Isayenkov, S. V. & Maathuis, F. J. M. Plant salinity stress: many unanswered questions remain. Front. Plant Sci. 10, 80 (2019).
Yang, Y. & Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60, 796–804 (2018).
Zhao, S. et al. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 22, 4609 (2021).
Liang, W., Ma, X., Wan, P. & Liu, L. Plant salt-tolerance mechanism: a review. Biochem. Biophys. Res. Commun. 495, 286–291 (2018).
Tanveera, M., Shahzada, B., Sharmac, A., Bijub, S. & Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: a review. Plant Physiol. Biochem. 130, 69–79 (2018).
Zhan, H. et al. Melatonin: a small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 20, 709 (2019).
Wu, H. Plant salt tolerance and Na+ sensing and transport. Crop J. 6, 215–225 (2018).
Zulfiqar, F. & Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 160, 257–268 (2021).
Per, T. S. et al. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 115, 126–140 (2017).
Yu, Z. et al. How plant hormones mediate salt stress responses.Trends Plant Sci. 25, 1117–1130 (2020).
Wang, C.-F. et al. Plant salinity sensors: current understanding and future directions. Front. Plant Sci. 13, 859224 (2022).
Yang, Y. & Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 217, 523–539 (2017).
Sanchez, D. H. et al. Integrative functional genomics of salt acclimatization in the model legume Lotus japonicus. Plant J. 53, 973–987 (2008).
Zeiner, M. et al. Influence of soil salinity on selected element contents in different brassica species. Molecules 27, 1878 (2022).
Khan, M. I. R., Asgher, M. & Khan, N. A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 80, 67–74 (2014).
Chen, Y.-E. et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plantarum 164, 349–363 (2018).
Sehar, Z., Masood, A. & Khan, N. A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 161, 277–289 (2019).
Park, M., Lee, H., Lee, J.-S., Byun, M.-O. & Kim, B.-G. In planta measurements of Na+ using fluorescent dye CoroNa Green. J. Plant Biol. 52, 298–302 (2009).
Wu, H. et al. Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Front. Plant Sci. 6, 71 (2015).
Cuin, T. A. et al. Assessing the role of root plasma membrane andtonoplast Na+/H+exchangers in salinity tolerancein wheat: in planta quantification methods. Plant Cell Environ. 34, 947–949 (2011).
Wu, H. et al. Developing and validating a high-throughput assay for salinity tissue tolerance in wheat and barley. Planta 242, 847–857 (2015).
Zhang, Y. et al. Copalyl diphosphate synthase mutation improved salt tolerance in maize (Zea mays. L) via enhancing vacuolar Na+ sequestration and maintaining ROS homeostasis. Front. Plant Sci. 11, 457 (2020).
Wu, H. et al. Na+ extrusion from the cytosol and tissue-specific Na sequestration in roots confer differential salt stress tolerance between durum and bread wheat. J. Exp. Bot. 69, 3987–4001 (2018).
Pedersen, O., Revsbech, N. P. & Shabala, S. Microsensors in plant biology: in vivo visualization of inorganic analytes with high spatial and/or temporal resolution. J. Exp. Bot. 71, 3941–3954 (2020).
Liu, K. et al. Application of non-invasive microelectrode ion flux estimation technique in crop stress physiology. Chin. J. Appl. Ecol. 29, 678–686 (2018).
Shabala, S., Shabala, L., Bose, J., Cuin, T. & Newman, I. Ion flux measurements using the MIFE technique. Methods Mol. Bio. 953, 171–183 (2013).
Martin, V. V. & Gee, A. R. R. Fluorescent metal ion indicators based on benzoannelated crown systems: a green fluorescent indicator for intracellular sodium ions. Bioorg. Med. Chem. Lett. 15, 1851–1855 (2005).
Yan, C. et al. Preparation of near-infrared AIEgen-active fluorescent probes for mapping amyloid-β plaques in brain tissues and living mice. Nat. Protoc. 18, 1316–1336 (2023).
Yin, J. et al. Preparation of a cyanine-based fluorescent probe for highly selective detection of glutathione and its use in living cells and tissues of mice. Nat. Protoc. 10, 1742–1754 (2015).
Gadella, T. W. J. New near-infrared fluorescent probes and tools. Nat. Methods 19, 654–655 (2022).
Liu, Y. et al. A cyanine dye to probe mitophagy: simultaneous detection of mitochondria and autolysosomes in live cells. J. Am. Chem. Soc. 138, 12368–12374 (2016).
Sun, W. et al. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 116, 7768–7817 (2016).
Shen, S. et al. Recent progress on fluorescent probes for viruses. Chin. Chem. Lett. 35, 108360 (2024).
Huang, Y., Ma, X., Li, J., Tan, C. & Yin, J. NIR-II cyanine nanoparticles for imaging-guided tumor targeting photothermal therapy with vitamin C enhanced efficacy. Adv. Therapeutics 6, 2300017 (2023).
Li, D. et al. Synergistic effects of multiple rotors and hydrogen-bond interactions lead to sensitive near-infrared viscosity probes for live-cell microscopy. Sci. China Chem. 66, 2329–2338 (2023).
Bourceau, P. et al. Visualization of metabolites and microbes at high spatial resolution using MALDI mass spectrometry imaging and in situ fluorescence labeling. Nat. Protoc. 18, 3050–3079 (2023).
Michaluk, P. & Rusakov, D. A. Monitoring cell membrane recycling dynamics of proteins using whole-cell fluorescence recovery after photobleaching of pH-sensitive genetic tags. Nat. Protoc. 17, 3056–3079 (2022).
Zeng, X. et al. Design of an HPPD fluorescent probe and visualization of plant responses to abiotic stress. Adv. Agrochem. 1, 73–84 (2022).
Zeng, X. et al. Fluorescence probes for reactive sulfur species in agricultural chemistry. J. Agric. Food Chem. 69, 13700–13712 (2021).
Zeng, X. et al. A protocol for activated bioorthogonal fluorescence labeling and imaging of 4-hydroxyphenylpyruvate dioxygenase in plants. Angew. Chem. Int. Ed. 62, e202312618 (2023).
Li, J., Yim, D., Jang, W.-D. & Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 46, 2437–2458 (2017).
Yin, J., Hua, Y. & Yoon, J. Fluorescent probes and bioimaging: alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 44, 4619–4644 (2015).
Chen, Y., Zheng, S., Kim, M. H., Chen, X. & Yoon, J. Recent progress of TP/NIR fluorescent probes for metal ions. Curr. Opin. Chem. Biol. 75, 102321 (2023).
Aron, A. T., Ramos-Torres, K. M., Cotruvo, J. A. Jr. & Chang, C. J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 48, 2434–2442 (2015).
Qiao, M., Ding, L. & Lv, F. Surfactant assemblies encapsulating fluorescent probes as selective and discriminative sensors for metal ions. Coord. Chem. Rev. 432, 213696 (2021).
Hamilton, G. R. C. et al. Optical probes for the detection of protons, and alkali and alkaline earth metal cations. Chem. Soc. Rev. 44, 4415–4432 (2015).
Zhao, M. et al. Supramolecularly engineered NIR-II and upconversion nanoparticles in vivo assembly and disassembly to improve bioimaging. Adv. Mater. 30, 1804982 (2018).
Zhao, M., Li, B., Fan, Y. & Zhang, F. In vivo assembly and disassembly of probes to improve near-infrared optical bioimaging. Adv. Healthc. Mater. 8, 1801650 (2019).
Ma, X. et al. The aggregates of near-infrared cyanine dyes in phototherapy. ChemMedChem 18, e202300204 (2023).
Chen, W. et al. Shortwave infrared imaging with J-aggregates stabilized in hollow mesoporous silica nanoparticles. J. Am. Chem. Soc. 141, 12475–12480 (2019).
Sun, C. et al. J-Aggregates of cyanine dye for NIR-II in vivo dynamic vascular imaging beyond 1,500 nm. J. Am. Chem. Soc. 141, 19221–19225 (2019).
Wood, C. A. et al. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat. Commun. 12, 5410 (2021).
Cao, W. & Sletten, E. M. fluorescent cyanine dye J-aggregates in the fluorous phase. J. Am. Chem. Soc. 140, 2727–2730 (2018).
Deng, X. et al. In vivo deep-brain 2-photon fluorescent microscopy labeled with near-infrared dyes excited at the 1,700 nm window. Anal. Chim. Acta 1255, 341118 (2023).
Ma, X. Construction and bioimaging application of novel indole heptamethine cyanines containing functionalized tetrahydropyridine rings. J. Mater. Chem. B 8, 9906–9912 (2020).
Ma, X. et al. J-Aggregates formed by NaCl treatment of Aza-coating heptamethine cyanines and their application to monitoring salt stress of plants and promoting photothermal therapy of tumors. Angew. Chem. Int. Ed. 62, e202216109 (2023).
Wu, H. et al. Monitoring plant health with near-infrared fluorescent H2O2 nanosensors. Nano Lett. 20, 2432–2442 (2020).
Xu, Z. et al. A visible and near-infrared, dual-channel fluorescence-on probe for selectively tracking mitochondrial glutathione. Chem 7, 1609–1628 (2018).
Liao, Y. et al. Heptamethine cyanines in bioorthogonal chemistry. Chin. Chem. Lett. 35, 109092 (2024).
Ma, X. et al. Rational design and application of an indolium-derived heptamethine cyanine with record-long second near-infrared emission. CCS Chem. 6, 1961–1976 (2022).
Liang, X., Li, J., Yang, Y., Jiang, C. & Guo, Y. Designing salt stress-resilient crops: current progress and future challenges. J. Integr. Plant Biol. 66, 303–329 (2024).
Zhang, Y. et al. Structural basis for the activity regulation of Salt Overly Sensitive 1 in Arabidopsis salt tolerance. Nat. Plants 9, 1915–1923 (2023).
Zhang, X.-Y. et al. Structure and activation mechanism of the rice Salt Overly Sensitive 1 (SOS1) Na/H antiporter. Nat. Plants 9, 1924–1936 (2023).
Liu, G. et al. SOS2 phosphorylates FREE1 during salt stress influencing MVB traffic and ultimately vacuole dynamics. Plant Cell (in the press).
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFA1207400), National Natural Science Foundation of China (22274061), the 111 Project (B17019) and Fundamental Research Funds for the Central Universities (CCNU24JCPT030). We would like to thank Y. Guo and G. Liu from China Agricultural University and H. Wu and J. Li from Huazhong Agricultural University for their guidance and assistance.
Author information
Authors and Affiliations
Contributions
All the experiments were conducted and the manuscript was written by X.M. with the supervision of S.H.L., J.Y. and G.-F.Y. X.Z. and Y.H. contributed to the manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Sergey Shabala and the other, anonymous, reviewer(s) for their contribution to the peer review process of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Ma, X. et al. Angew. Chem. Int. Ed. 62, e202216109 (2023): https://doi.org/10.1002/anie.202216109
Supplementary information
Supplementary Information
Supplementary Figs. 1–36, and Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, X., Zeng, X., Huang, Y. et al. Visualizing plant salt stress with a NaCl-responsive fluorescent probe. Nat Protoc 20, 902–933 (2025). https://doi.org/10.1038/s41596-024-01068-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01068-x