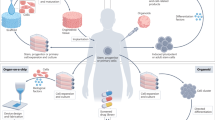
Abstract
Organoids are in vitro miniaturized cellular models of organs that offer opportunities for studying organ development, disease mechanisms and drug screening. Understanding the complex processes governing organoid development and function requires methods suitable for the continuous, long-term monitoring of cell activities (for example, electrophysiological and mechanical activity) at single-cell resolution throughout the entire three-dimensional (3D) structure. Cyborg organoid technology addresses this need by seamlessly integrating stretchable mesh nanoelectronics with tissue-like properties, such as tissue-level flexibility, subcellular feature size and mesh-like networks, into 3D organoids through a 2D-to-3D tissue reconfiguration process during organogenesis. This approach enables longitudinal, tissue-wide, single-cell functional mapping, thereby overcoming the limitations of existing techniques including recording duration, spatial coverage, and the ability to maintain stable contact with the tissue during organoid development. This protocol describes the fabrication and characterization of stretchable mesh nanoelectronics, their electrical performance, their integration with organoids and the acquisition of long-term functional organoid activity requiring multimodal data analysis techniques. Cyborg organoid technology represents a transformative tool for investigating organoid development and function, with potential for improving in vitro disease models, drug screening and personalized medicine. The procedure is suitable for users within a multidisciplinary team with expertise in stem cell biology, tissue engineering, nanoelectronics fabrication, electrophysiology and data science.
Key points
-
The procedure includes the fabrication of stretchable mesh nanoelectronics, the nanoelectronics’ integration within developing organoids and the long-term electrical measurements of organoid function.
-
Alternatives include imaging-based techniques, intracellular recordings and extracellular recordings using multielectrode arrays. Cyborg organoids provide stable 3D bioelectronics interfaces and enable the continuous monitoring of electrophysiological activity during development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the main data discussed in this protocol are available in the supporting primary research papers (https://doi.org/10.1021/acs.nanolett.9b02512, https://doi.org/10.1002/adma.202106829, https://doi.org/10.1126/sciadv.ade8513, and https://doi.org/10.1016/j.cell.2023.03.023, https://doi.org/10.1101/2024.03.18.585551).
References
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell. Biol. 21, 571–584 (2020).
Zhao, Z. et al. Organoids. Nat. Rev. Methods Primers 2, 94 (2022).
Li, Q. et al. Cyborg organoids: implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett. 19, 5781–5789 (2019).
Le Floch, P. et al. Stretchable mesh nanoelectronics for 3D single-cell chronic electrophysiology from developing brain organoids. Adv. Mater. 34, e2106829 (2022).
Lin, Z. et al. Tissue-embedded stretchable nanoelectronics reveal endothelial cell-mediated electrical maturation of human 3D cardiac microtissues. Sci. Adv. 9, eade8513 (2023).
Li, Q. et al. Multimodal charting of molecular and functional cell states via in situ electro-sequencing. Cell 186, 2002–2017 e2021 (2023).
Wurst, W. & Bally-Cuif, L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat. Rev. Neurosci. 2, 99–108 (2001).
Harvey, R. P. Patterning the vertebrate heart. Nat. Rev. Genet. 3, 544–556 (2002).
Li, Q. et al. Cyborg islets: implanted flexible electronics reveal principles of human islet electrical maturation. Preprint at bioRxiv 2024.2003.2018.585551 (2024).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Liu, J. et al. Syringe-injectable electronics. Nat Nanotechnol. 10, 629–636 (2015).
Kim, D. H., Ghaffari, R., Lu, N. & Rogers, J. A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 14, 113–128 (2012).
Tang, X., Shen, H., Zhao, S., Li, N. & Liu, J. Flexible brain–computer interfaces. Nat. Electron. 6, 109–118 (2023).
Fan, J. A. et al. Fractal design concepts for stretchable electronics. Nat. Commun. 5, 3266 (2014).
Xu, S. et al. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling. Science 347, 154–159 (2015).
Feiner, R. et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 15, 679–685 (2016).
Feiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2017).
Fu, T. M., Hong, G., Viveros, R. D., Zhou, T. & Lieber, C. M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl Acad. Sci. USA 114, E10046–E10055 (2017).
Gao, H. et al. Graphene-integrated mesh electronics with converged multifunctionality for tracking multimodal excitation-contraction dynamics in cardiac microtissues. Nat. Commun. 15, 2321 (2024).
Quadrato, G., Brown, J. & Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 22, 1220–1228 (2016).
Smirnova, L. Biocomputing with organoid intelligence. Nat. Rev. Bioeng. 2, 633–634 (2024).
Kagan, B. J. et al. In vitro neurons learn and exhibit sentience when embodied in a simulated game-world. Neuron 110, 3952–3969 e3958 (2022).
Cai, H. et al. Brain organoid reservoir computing for artificial intelligence. Nat. Electron. 6, 1032–1039 (2023).
Chung, W. G. et al. Recent advances in electrophysiological recording platforms for brain and heart organoids. Adv. NanoBiomed Res. 2, 2200081 (2022).
Richards, D. J. et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 4, 446–462 (2020).
Sakaguchi, H. et al. Self-organized synchronous calcium transients in a cultured human neural network derived from cerebral organoids. Stem Cell Reports 13, 458–473 (2019).
Yamamoto, Y., Hirose, S., Wuriyanghai, Y., Yoshinaga, D. & Makiyama, T. Electrophysiological analysis of hiPSC-derived cardiomyocytes using a patch-clamp technique. Methods Mol. Biol. 2320, 121–133 (2021).
Xiang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398 e387 (2017).
Lewis-Israeli, Y. R. et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 12, 5142 (2021).
Negraes, P. D. et al. Altered network and rescue of human neurons derived from individuals with early-onset genetic epilepsy. Mol. Psychiatry 26, 7047–7068 (2021).
Velasco, S. et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527 (2019).
Di Lullo, E. & Kriegstein, A. R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18, 573–584 (2017).
Chung, J. E. et al. A fully automated approach to spike sorting. Neuron 95, 1381–1394 e1386 (2017).
Pachitariu, M., Sridhar, S., Pennington, J. & Stringer, C. Spike sorting with Kilosort4. Nat Methods 21, 914–921 (2024).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529 (2021).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA 109, E1848–E1857 (2012).
Yoon, S. J. et al. Reliability of human cortical organoid generation. Nat. Methods 16, 75–78 (2019).
Pollock, S. D., Galicia-Silva, I. M., Liu, M., Gruskin, Z. L. & Alvarez-Dominguez, J. R. Scalable generation of 3D pancreatic islet organoids from human pluripotent stem cells in suspension bioreactors. STAR Protoc. 4, 102715 (2023).
Alvarez-Dominguez, J. R. et al. Circadian entrainment triggers maturation of human in vitro islets. Cell Stem Cell 26, 108–122 e110 (2020).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. Elife 9, e61834 (2020).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2018).
Moon, K. R. et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 37, 1482–1492 (2019).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Gala, R. et al. Consistent cross-modal identification of cortical neurons with coupled autoencoders. Nat. Comput. Sci. 1, 120–127 (2021).
Liu, R. et al. An AI-cyborg system for adaptive intelligent modulation of organoid maturation. Preprint at bioRxiv https://doi.org/10.1101/2024.12.07.627355 (2024).
Acknowledgements
We acknowledge the valuable discussions with A.ML., X.Z. and H.S. J.Liu acknowledges the support of NIH/NIMH 1RF1MH123948; NIH/NIDDK 1DP1DK130673; NSF/ECCS-2038603; NIH/NLM 5R01LM014465.
Author information
Authors and Affiliations
Contributions
Z.L., W.W., R.L., Q.L. and J.Liu. developed the protocol and drafted the manuscript with input from C.H. and J.Lee. All authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
J. Liu is a co-founder of Axoft, Inc.
Peer review
Peer review information
Nature Protocols thanks Gi Doo Cha and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Li, Q. et al. Nano Lett. 19, 5781–5789 (2019): https://doi.org/10.1021/acs.nanolett.9b02512
Lin, Z. et al. Sci. Adv. 9, eade8513 (2023): https://doi.org/10.1126/sciadv.ade8513
Li, Q. et al. Cell 186, 2002–2017 e2021 (2023): https://doi.org/10.1016/j.cell.2023.03.023
Le Floch, P. et al. Adv. Mater. 34, e2106829 (2022): https://doi.org/10.1002/adma.202106829
Supplementary information
Supplementary Information
Supplementary Figures 1-2.
Supplementary Data 1
Photomask of mesh nanoelectronics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, Z., Wang, W., Liu, R. et al. Cyborg organoids integrated with stretchable nanoelectronics can be functionally mapped during development. Nat Protoc 20, 2528–2559 (2025). https://doi.org/10.1038/s41596-025-01147-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-025-01147-7