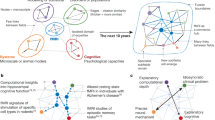
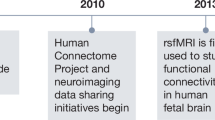
Abstract
Concurrent transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (TMS-fMRI) provides a step-change in the toolkit of neuroscience research. TMS enables the noninvasive perturbation of ongoing human brain activity, and when coupled to fMRI for the simultaneous read-out of its effects across the brain, concurrent TMS-fMRI enables studies aimed at determining the causal inference of human brain–behavior relationships, with implications for both fundamental research and clinical application. Many of the technical barriers to TMS-fMRI implementation, such as hardware design and setups, have now been overcome, and the research community in the field is rapidly growing. Here, we present the guidelines set by an international consensus, from researchers at all levels and across the fields of cognitive and applied human neuroscience, for the experimental design and practical considerations of concurrent TMS-fMRI via 12 detailed use cases. These guidelines may facilitate the uptake of this approach and simplify the experimental design and planning stages.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Driver, J., Blankenburg, F., Bestmann, S., Vanduffel, W. & Ruff, C. C. Concurrent brain-stimulation and neuroimaging for studies of cognition. Trends Cogn. Sci. 13, 319–327 (2009).
Bergmann, T. O. et al. Concurrent TMS-fMRI for causal network perturbation and proof of target engagement. Neuroimage 237, 118093 (2021).
Feredoes, E., Tononi, G. & Postle, B. R. The neural bases of the short-term storage of verbal information are anatomically variable across individuals. J. Neurosci. 27, 11003–11008 (2007).
Sack, A. T. et al. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J. Cogn. Neurosci. 21, 207–221 (2009).
Sack, A. T., Kohler, A., Linden, D. E., Goebel, R. & Muckli, L. The temporal characteristics of motion processing in hMT/V5+: combining fMRI and neuronavigated TMS. Neuroimage 29, 1326–1335 (2006).
Hebscher, M. & Voss, J. L. Testing network properties of episodic memory using non-invasive brain stimulation. Curr. Opin. Behav. Sci. 32, 35–42 (2020).
Wang, J. X. et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 345, 1054–1057 (2014).
Tambini, A., Nee, D. E. & D’Esposito, M. Hippocampal-targeted theta-burst stimulation enhances associative memory formation. J. Cogn. Neurosci. 30, 1452–1472 (2018).
Beynel, L. et al. Online repetitive transcranial magnetic stimulation during working memory in younger and older adults: a randomized within-subject comparison. PLoS One 14, e0213707 (2019).
Dave, S., VanHaerents, S., Bonakdarpour, B., Mesulam, M. M. & Voss, J. L. Stimulation of distinct parietal locations differentiates frontal versus hippocampal network involvement in memory formation. Curr. Res. Neurobiol. 3, 100030 (2022).
Dave, S., VanHaerents, S. & Voss, J. L. Cerebellar theta and beta noninvasive stimulation rhythms differentially influence episodic memory versus semantic prediction. J. Neurosci. 40, 7300–7310 (2020).
Hermiller, M. S. et al. Evidence from theta-burst stimulation that age-related de-differentiation of the hippocampal network is functional for episodic memory. Neurobiol. Aging 109, 145–157 (2022).
Hermiller, M. S., VanHaerents, S., Raij, T. & Voss, J. L. Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity. Hippocampus 29, 595–609 (2019).
Eldaief, M. C., Halko, M. A., Buckner, R. L. & Pascual-Leone, A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl Acad. Sci. USA 108, 21229–21234 (2011).
Gratton, C., Lee, T. G., Nomura, E. M. & D’Esposito, M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front. Syst. Neurosci. 7, 124 (2013).
Friehs, M. A. et al. No effects of 1 Hz offline TMS on performance in the stop-signal game. Sci. Rep. 13, 11565 (2023).
De Pisapia, N., Barchiesi, G., Jovicich, J. & Cattaneo, L. The role of medial prefrontal cortex in processing emotional self-referential information: a combined TMS/fMRI study. Brain Imaging Behav. 13, 603–614 (2019).
Min, Y. S. et al. Neuromodulatory effects of offline low-frequency repetitive transcranial magnetic stimulation of the motor cortex: a functional magnetic resonance imaging study. Sci. Rep. 6, 36058 (2016).
Petitet, P., Noonan, M. P., Bridge, H., O’Reilly, J. X. & O’Shea, J. Testing the inter-hemispheric competition account of visual extinction with combined TMS/fMRI. Neuropsychologia 74, 63–73 (2015).
Hoogendam, J. M., Ramakers, G. M. & Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118 (2010).
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P. & Rothwell, J. C. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (2005).
Wischnewski, M. & Schutter, D. J. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 8, 685–692 (2015).
Rossi, S. et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 132, 269–306 (2021).
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A. & Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039 (2009).
Tik, M. et al. Acute TMS/fMRI response explains offline TMS network effects—An interleaved TMS-fMRI study. Neuroimage 267, 119833 (2023).
Tik, M. et al. Concurrent TMS/fMRI reveals individual DLPFC dose-response pattern. Neuroimage 282, 120394 (2023).
Rafiei, F. & Rahnev, D. TMS does not increase BOLD activity at the site of stimulation: a review of all concurrent TMS-fMRI studies. eNeuro 9, ENEURO.0163-22.2022 (2022).
Rafiei, F., Safrin, M., Wokke, M. E., Lau, H. & Rahnev, D. Transcranial magnetic stimulation alters multivoxel patterns in the absence of overall activity changes. Hum. Brain Mapp. 42, 3804–3820 (2021).
Bergmann, T. O. & Hartwigsen, G. Inferring causality from noninvasive brain stimulation in cognitive neuroscience. J. Cogn. Neurosci. 33, 195–225 (2021).
Oathes, D. J. et al. Resting fMRI-guided TMS results in subcortical and brain network modulation indexed by interleaved TMS/fMRI. Exp. Brain Res. 239, 1165–1178 (2021).
Vink, J. J. T. et al. A novel concurrent TMS-fMRI method to reveal propagation patterns of prefrontal magnetic brain stimulation. Hum. Brain Mapp. 39, 4580–4592 (2018).
Oathes, D. J. et al. Non-invasively targeting, probing and modulating a deep brain circuit for depression alleviation. Nat. Ment. Health 1, 1033–1042 (2023).
Grosshagauer, S. et al. Chronometric TMS-fMRI of personalized left dorsolateral prefrontal target reveals state-dependency of subgenual anterior cingulate cortex effects. Mol. Psychiatry 29, 2678–2688 (2024).
Sydnor, V. J. et al. Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala. Sci. Adv. 8, eabn5803 (2022).
Hermiller, M. S., Chen, Y. F., Parrish, T. B. & Voss, J. L. Evidence for immediate enhancement of hippocampal memory encoding by network-targeted theta-burst stimulation during concurrent fMRI. J. Neurosci. 40, 7155–7168 (2020).
Bestmann, S., Baudewig, J., Siebner, H. R., Rothwell, J. C. & Frahm, J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage 20, 1685–1696 (2003).
Goldenkoff, E. R. et al. The behavioral and neural effects of parietal theta burst stimulation on the grasp network are stronger during a grasping task than at rest. Front. Neurosci. 17, 1198222 (2023).
Jannati, A., Oberman, L. M., Rotenberg, A. & Pascual-Leone, A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology 48, 191–208 (2023).
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H. & Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 133, 425–430 (2000).
Thickbroom, G. W. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp. Brain Res. 180, 583–593 (2007).
Hawco, C. et al. Differing time of onset of concurrent TMS-fMRI during associative memory encoding: a measure of dynamic connectivity. Front. Hum. Neurosci. 11, 404 (2017).
Feredoes, E., Heinen, K., Weiskopf, N., Ruff, C. & Driver, J. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl Acad. Sci. USA 108, 17510–17515 (2011).
Buzsaki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Janssens, S. E. W., Oever, S. T., Sack, A. T. & de Graaf, T. A. “Broadband Alpha Transcranial Alternating Current Stimulation”: exploring a new biologically calibrated brain stimulation protocol. Neuroimage 253, 119109 (2022).
Nelli, S., Itthipuripat, S., Srinivasan, R. & Serences, J. T. Fluctuations in instantaneous frequency predict alpha amplitude during visual perception. Nat. Commun. 8, 2071 (2017).
Vinck, M. et al. Principles of large-scale neural interactions. Neuron 111, 987–1002 (2023).
Dunlop, K. & Nestor, S. Comparing DLPFC 10 Hz and theta burst stimulation using interleaved TMS/fMRI. Brain Stimul. 16, 178 (2023).
Wassermann, E. M. in Oxford Handbook of Transcranial Stimulation (eds. Epstein, C. M., Wassermann, E. M. & Ziemann, U.) 401–408 (Oxford Academic, 2012).
Schilberg, L., Schuhmann, T. & Sack, A. T. Interindividual variability and intraindividual reliability of intermittent theta burst stimulation-induced neuroplasticity mechanisms in the healthy brain. J. Cogn. Neurosci. 29, 1022–1032 (2017).
Rocchi, L. et al. Variability and predictors of response to continuous theta burst stimulation: a TMS-EEG study. Front. Neurosci. 12, 400 (2018).
Bestmann, S. et al. Mapping causal interregional influences with concurrent TMS-fMRI. Exp. Brain Res. 191, 383–402 (2008).
Moisa, M., Siebner, H. R., Pohmann, R. & Thielscher, A. Uncovering a context-specific connectional fingerprint of human dorsal premotor cortex. J. Neurosci. 32, 7244–7252 (2012).
Sack, A. T. et al. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb. Cortex 17, 2841–2852 (2007).
Ruff, C. C. et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr. Biol. 16, 1479–1488 (2006).
Ruff, C. C. et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb. Cortex 18, 817–827 (2008).
Blankenburg, F. et al. Studying the role of human parietal cortex in visuospatial attention with concurrent TMS-fMRI. Cereb. Cortex 20, 2702–2711 (2010).
Heinen, K., Feredoes, E., Weiskopf, N., Ruff, C. C. & Driver, J. Direct evidence for attention-dependent influences of the frontal eye-fields on feature-responsive visual cortex. Cereb. Cortex 24, 2815–2821 (2014).
Peters, J. C. et al. Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity. Commun. Biol. 3, 40 (2020).
Silvanto, J. & Pascual-Leone, A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 21, 1–10 (2008).
Donse, L., Padberg, F., Sack, A. T., Rush, A. J. & Arns, M. Simultaneous rTMS and psychotherapy in major depressive disorder: clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 11, 337–345 (2018).
Neacsiu, A. D. et al. Enhancing cognitive restructuring with concurrent fMRI-guided neurostimulation for emotional dysregulation—A randomized controlled trial. J. Affect. Disord. 301, 378–389 (2022).
Neacsiu, A. D. et al. Enhancing cognitive restructuring with concurrent repetitive transcranial magnetic stimulation: a transdiagnostic randomized controlled trial. Psychother. Psychosom. 91, 94–106 (2022).
Neacsiu, A. D. et al. On the concurrent use of self-system therapy and functional magnetic resonance imaging-guided transcranial magnetic stimulation as treatment for depression. J. ECT 34, 266–273 (2018).
Petrov, P. I. et al. Validating models of TMS effects with concurrent TMS/fMRI. Preprint at https://www.biorxiv.org/content/10.1101/2023.07.05.547836v1 (2023).
Cocchi, L. et al. Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics. J. Neurophysiol. 113, 3375–3385 (2015).
Ge, R. et al. Predictive value of acute neuroplastic response to rTMS in treatment outcome in depression: a concurrent TMS-fMRI trial. Am. J. Psychiatry 179, 500–508 (2022).
Fonzo, G. A. et al. Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am. J. Psychiatry 174, 1175–1184 (2017).
Oathes, D. J. et al. Combining transcranial magnetic stimulation with functional magnetic resonance imaging for probing and modulating neural circuits relevant to affective disorders. Wiley Interdiscip. Rev. Cogn. Sci. 12, e1553 (2021).
Baudewig, J., Paulus, W. & Frahm, J. Artifacts caused by transcranial magnetic stimulation coils and EEG electrodes in T2*-weighted echo-planar imaging. Magn. Reson. Imaging 18, 479–484 (2000).
Bestmann, S., Baudewig, J. & Frahm, J. On the synchronization of transcranial magnetic stimulation and functional echo-planar imaging. J. Magn. Reson. Imaging 17, 309–316 (2003).
Bohning, D. E. et al. Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. Neuroreport 8, 2535–2538 (1997).
Navarro de Lara, L. I. et al. A novel coil array for combined TMS/fMRI experiments at 3 T. Magn. Reson. Med. 74, 1492–1501 (2015).
Hassan, U., Pillen, S., Zrenner, C. & Bergmann, T. O. The Brain Electrophysiological recording & STimulation (BEST) toolbox. Brain Stimul. 15, 109–115 (2022).
Riddle, J. et al. A guide for concurrent TMS-fMRI to investigate functional brain networks. Front. Hum. Neurosci. 16, 1050605 (2022).
Mizutani-Tiebel, Y. et al. Concurrent TMS-fMRI: technical challenges, developments, and overview of previous studies. Front. Psychiatry 13, 825205 (2022).
Bungert, A., Chambers, C. D., Long, E. & Evans, C. J. On the importance of specialized radiofrequency filtering for concurrent TMS/MRI. J. Neurosci. Methods 210, 202–205 (2012).
Weiskopf, N. et al. Image artifacts in concurrent transcranial magnetic stimulation (TMS) and fMRI caused by leakage currents: modeling and compensation. J. Magn. Reson. Imaging 29, 1211–1217 (2009).
Assem, M., Mada, M., Eldridge, S. & Woolgar, A. The Taco Setup: A Novel TMS-fMRI Setup for High Resolution Whole Brain Imaging. Preprint at https://www.biorxiv.org/content/10.1101/2025.06.14.659622v1 (2025).
Tik, M. Imaging therapeutic brain stimulation: translating technical innovations to inform treatment. In 2nd International Workshop on Concurrent TMS-fMRI (Greece, 2023).
Jackson, J. B., Scrivener, C. L., Mada, M. & Woolgar, A. Comparison of MR coil options for concurrent TMS-fMRI. Preprint at https://www.biorxiv.org/content/10.1101/2024.05.15.594454v1 (2024).
Navarro de Lara, L. I. et al. A novel whole-head RF coil design tailored for concurrent multichannel brain stimulation and imaging at 3T. Brain Stimul. 16, 1021–1031 (2023).
Dannhauer, M. et al. TAP: targeting and analysis pipeline for optimization and verification of coil placement in transcranial magnetic stimulation. J. Neural Eng. 19, https://doi.org/10.1088/1741-2552/ac63a4 (2022).
Gomez, L. J., Dannhauer, M. & Peterchev, A. V. Fast computational optimization of TMS coil placement for individualized electric field targeting. Neuroimage 228, 117696 (2021).
Numssen, O. et al. Efficient high-resolution TMS mapping of the human motor cortex by nonlinear regression. Neuroimage 245, 118654 (2021).
Lueckel, M. et al. Functional connectivity- and E-field-optimized TMS targeting: method development and concurrent TMS-fMRI validation. Brain Stimul. 16, 189 (2023).
Numssen, O., van der Burght, C. L. & Hartwigsen, G. Revisiting the focality of non-invasive brain stimulation—implications for studies of human cognition. Neurosci. Biobehav. Rev. 149, 105154 (2023).
Lynch, C. J. et al. Automated optimization of TMS coil placement for personalized functional network engagement. Neuron 110, 3263–3277.e4 (2022).
Lee, H. J., Woudsma, K. J., Ishraq, M. F. & Lin, F. H. Design of coil holder for the improved maneuvering in concurrent TMS-MRI. Brain Stimul. 16, 966–968 (2023).
Halko, M. A., Eldaief, M. C. & Pascual-Leone, A. Noninvasive brain stimulation in the study of the human visual system. J. Glaucoma 22, S39–S41 (2013).
Cerins, A. et al. A new angle on transcranial magnetic stimulation coil orientation: a targeted narrative review. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 9, 744–753 (2024).
Opitz, A., Fox, M. D., Craddock, R. C., Colcombe, S. & Milham, M. P. An integrated framework for targeting functional networks via transcranial magnetic stimulation. Neuroimage 127, 86–96 (2016).
Siebner, H. R. et al. Transcranial magnetic stimulation of the brain: what is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 140, 59–97 (2022).
Goldstein, S., Rafiei, F. & Rahnev, D. 3D-printed stand, timing interface, and coil localization tools for concurrent TMS-fMRI experiments. Brain Stimul. 15, 1290–1291 (2022).
Sparing, R., Buelte, D., Meister, I. G., Paus, T. & Fink, G. R. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum. Brain Mapp. 29, 82–96 (2008).
Grodzki, D. M., Jakob, P. M. & Heismann, B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn. Reson. Med. 67, 510–518 (2012).
Herwig, U. et al. The navigation of transcranial magnetic stimulation. Psychiatry Res. 108, 123–131 (2001).
Windischberger, C., Woletz, M., Grosshagauer, S., Vasileiadi, M. & Tik, M. Neuronavigation-based improvements of concurrent TMS/fMRI studies. Brain Stimul. 16, 193 (2023).
McKeown, M. J., Hansen, L. K. & Sejnowsk, T. J. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol. 13, 620–629 (2003).
Windischberger, C., Tik, M., Thielscher, A. & Siebner, H. R. Transcranial brain stimulation and functional MRI. In The Oxford Handbook of Transcranial Stimulation (eds. Wassermann, E. M. et al.) 648–674 (Oxford Academic, 2022).
Bestmann, S., Ruff, C. C., Driver, J. & Blankenburg, F. Concurrent TMS and functional magnetic resonance imaging: methods and current advances. In Oxford Handbook of Transcranial Stimulation (Epstein, C. M., Wassermann, E. M. & Ziemann, U.) 569–592 (Oxford Academic, 2008).
Jackson, J. B., Feredoes, E., Rich, A. N., Lindner, M. & Woolgar, A. Concurrent neuroimaging and neurostimulation reveals a causal role for dlPFC in coding of task-relevant information. Commun. Biol. 4, 588 (2021).
Bestmann, S. et al. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb. Cortex 18, 1281–1291 (2008).
Bohning, D. E. et al. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest. Radiol. 33, 336–340 (1998).
Scrivener, C. L., Jackson, J. B., Correia, M. M., Mada, M. & Woolgar, A. Now you see it, now you don’t: optimal parameters for interslice stimulation in concurrent TMS-fMRI. Preprint at https://www.biorxiv.org/content/10.1101/2021.05.28.446111v1 (2021).
Moisa, M., Pohmann, R., Uludag, K. & Thielscher, A. Interleaved TMS/CASL: comparison of different rTMS protocols. Neuroimage 49, 612–620 (2010).
Valero-Cabre, A., Amengual, J. L., Stengel, C., Pascual-Leone, A. & Coubard, O. A. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci. Biobehav. Rev. 83, 381–404 (2017).
Habibollahi Saatlou, F. et al. MAGIC: an open-source MATLAB toolbox for external control of transcranial magnetic stimulation devices. Brain Stimul. 11, 1189–1191 (2018).
Klooster, D. C. W., Ferguson, M. A., Boon, P. & Baeken, C. Personalizing repetitive transcranial magnetic stimulation parameters for depression treatment using multimodal neuroimaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 536–545 (2022).
Turi, Z., Lenz, M., Paulus, W., Mittner, M. & Vlachos, A. Selecting stimulation intensity in repetitive transcranial magnetic stimulation studies: a systematic review between 1991 and 2020. Eur. J. Neurosci. 53, 3404–3415 (2021).
Stokes, M. G. et al. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J. Neurophysiol. 94, 4520–4527 (2005).
Caulfield, K. A., Li, X. & George, M. S. Four electric field modeling methods of Dosing Prefrontal Transcranial Magnetic Stimulation (TMS): introducing APEX MT dosimetry. Brain Stimul. 14, 1032–1034 (2021).
Numssen, O., Kuhnke, P., Weise, K. & Hartwigsen, G. Electric-field-based dosing for TMS. Imaging Neurosci. (Camb.) 2, 1–12 (2024).
Nee, D. E. fMRI replicability depends upon sufficient individual-level data. Commun. Biol. 2, 130 (2019).
Hawco, C., Steeves, J. K. E., Voineskos, A. N., Blumberger, D. M. & Daskalakis, Z. J. Within-subject reliability of concurrent TMS-fMRI during a single session. Psychophysiology 60, e14252 (2023).
Peters, J. C. et al. On the feasibility of concurrent human TMS-EEG-fMRI measurements. J. Neurophysiol. 109, 1214–1227 (2013).
Duecker, F. & Sack, A. T. Rethinking the role of sham TMS. Front. Psychol. 6, 210 (2015).
Duecker, F. & Sack, A. T. Pre-stimulus sham TMS facilitates target detection. PLoS One 8, e57765 (2013).
Bergmann, T. O., Karabanov, A., Hartwigsen, G., Thielscher, A. & Siebner, H. R. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: current approaches and future perspectives. Neuroimage 140, 4–19 (2016).
Heinen, K. et al. Concurrent TMS-fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. Eur. J. Neurosci. 33, 991–1000 (2011).
Leitao, J., Thielscher, A., Tunnerhoff, J. & Noppeney, U. Concurrent TMS-fMRI reveals interactions between dorsal and ventral attentional systems. J. Neurosci. 35, 11445–11457 (2015).
Schwarzkopf, D. S., Silvanto, J. & Rees, G. Stochastic resonance effects reveal the neural mechanisms of transcranial magnetic stimulation. J. Neurosci. 31, 3143–3147 (2011).
Giustiniani, A. et al. A questionnaire to collect unintended effects of transcranial magnetic stimulation: a consensus based approach. Clin. Neurophysiol. 141, 101–108 (2022).
Bestmann, S., Baudewig, J., Siebner, H. R., Rothwell, J. C. & Frahm, J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 28, 22–29 (2005).
O’Shea, J., Johansen-Berg, H., Trief, D., Gobel, S. & Rushworth, M. F. Functionally specific reorganization in human premotor cortex. Neuron 54, 479–490 (2007).
Hartwigsen, G. et al. Rapid short-term reorganization in the language network. eLife 6, e25964 (2017).
Jung, J., Bungert, A., Bowtell, R. & Jackson, S. R. Vertex stimulation as a control site for transcranial magnetic stimulation: a concurrent TMS/fMRI study. Brain Stimul. 9, 58–64 (2016).
Leitao, J., Thielscher, A., Tuennerhoff, J. & Noppeney, U. Comparing TMS perturbations to occipital and parietal cortices in concurrent TMS-fMRI studies—methodological considerations. PLoS One 12, e0181438 (2017).
de Graaf, T. A., Jacobs, C., Roebroeck, A. & Sack, A. T. FMRI effective connectivity and TMS chronometry: complementary accounts of causality in the visuospatial judgment network. PLoS One 4, e8307 (2009).
Bradley, C., Nydam, A. S., Dux, P. E. & Mattingley, J. B. State-dependent effects of neural stimulation on brain function and cognition. Nat. Rev. Neurosci. 23, 459–475 (2022).
Terney, D., Chaieb, L., Moliadze, V., Antal, A. & Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 28, 14147–14155 (2008).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Allen, E. A., Pasley, B. N., Duong, T. & Freeman, R. D. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science 317, 1918–1921 (2007).
Manohar, S., Whyte, C., Feredoes, E. & Woolgar, A. An architecture for zero-shot decision-making using plastic attractors. In Conference on Cognitive Computational Neuroscience, P-2B.119, 1333 (Oxford, 2023).
Coalson, T. S., Van Essen, D. C. & Glasser, M. F. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proc. Natl Acad. Sci. USA 115, E6356–E6365 (2018).
Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016).
Hawco, C. et al. Spread of activity following TMS is related to intrinsic resting connectivity to the salience network: a concurrent TMS-fMRI study. Cortex 108, 160–172 (2018).
Navarro de Lara, L. I. et al. High-sensitivity TMS/fMRI of the human motor cortex using a dedicated multichannel MR coil. Neuroimage 150, 262–269 (2017).
Chang, K. Y. et al. Neural response during prefrontal theta burst stimulation: interleaved TMS-fMRI of full iTBS protocols. Neuroimage 291, 120596 (2024).
Burke, M. et al. Improving coil setup and data processing strategies for concurrent (f)MRI and brain-stimulation studies. In Proceedings of the International Society for Magnetic Resonance in Medicine (Singapore, 2024).
Acknowledgements
This work was funded by a wide range of sources including salary and project support to the authors. These include UKRI MRC intramural funding (SUAG/093/G116768) and UKRI MRC Promote Units as National Assets Award (MC_PC_20046) to A.W.; a UKRI BBSRC grant (BB/S019170/1) to A.W. and M.M.C; a NWO-OC grant (406.20.GO.004) from the Dutch Research Council (NWO) to A.T.S.; research grants by the Deutsche Forschungsgemeinschaft (DFG (German Research Foundation) No. 525127358 and No. 525176435 to T.O.B. and HA 6314/4-2 and HA 6314/10-1 to G.H.); and the European Research Council (ERC-2021-COG 101043747) to G.H.; National Institutes of Health grants (R61 MH135428 and R01 MH120811) and funding from the Hart Fund in Cognitive Neuroscience and New Venture Fund/The Foundation for OCD Research to D.J.O.; Lise Meitner Excellence Funding by the Max Planck Society to G.H.; an Early Career Research Award from the Centre for Integrative Neuroscience Discovery, University of Cambridge to M.A.; funding from the Austrian Science Fund (P33180), Austrian Ministry for Science and Education (103HSK02) and European Commission (612022) to C.W.; the Federal Ministry of Education and Research (BMBF, grant no. 01GQ2201) supporting O.N.; funding from the NIMH Intramural Research Program (ZIAMH002955) supporting L.B. and B.L.; and funding from the Lundbeck Foundation (grants R313-2019-622 and R244-2017-196) and the Innovation Fund Denmark (Grand Solutions grant 9068-00025B) supporting A.T. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
This work arises from a series of consensus discussion meetings held over 3 days at an international workshop on TMS-fMRI organized by E.F., A.W., C.W. and M.T. in Greece in May–June 2023. The writing of this consensus paper was led by A.W., E.F., M.S.H. and A.T.S. A.W., E.F., M.A., Y.B., M.B., R.F.H.C., R.M.C., G.H., J.B.J., M.K., B.L., M.L., E.M., O.N., D.J.O., A.C.R., T.S., C.L.S., Y.T., M.V., C.W., M.S.H. and A.T.S. conceived the work. A.W., E.F., M.A., Y.B., L.B., R.M.C., G.H., J.B.J., M.K., P.K., O.L., M.L., C.M., E.M., O.N., D.J.O., C.L.S., M.S.H. and A.T.S. wrote the original draft. A.W., E.F., M.A., Y.B., T.O.B., M.B., R.F.H.C., M.M.C., E.G., G.H., J.B.J., M.K., B.L., M.L., E.M., O.N., D.J.O., A.-L.S., A.T., M.T., Y.T., M.V., C.W., M.S.H. and A.T.S. reviewed and edited the manuscript. A.W., E.F., M.A., Y.B., M.B., R.M.C., M.L., D.J.O. and M.S.H. visualized the work. A.W., E.F., M.S.H. and A.T.S. supervised the work. A.W. provided project administration.
Corresponding author
Ethics declarations
Competing interests
A.T.S. is Chief Scientific Advisor of PlatoScience Medical and Alpha Brain Technologies, Chief Executive Officer of Neurowear Medical and Director of the Clinical TMS Certification Course (www.tmscourse.eu) and received equipment support from MagVenture, MagStim and Deymed. C.W. is a member of the scientific advisory board of Brightmind.AI GmbH and a shareholder of ALSIX GmbH, supplier of tailored MR hardware. P.K. is employed by Localite GmbH, manufacturer of TMS neuronavigation devices. M.K., C.M. and Y.T. are employees of MagVenture, suppliers of TMS-fMRI solutions. R.M.C. is an employee of Rogue Research Solutions, supplier of TMS neuronavigation devices.
Peer review
Peer review information
Nature Protocols thanks Jeyoung Jung and Kevin Caulfield for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woolgar, A., Feredoes, E., Assem, M. et al. Consensus guidelines for the use of concurrent TMS-fMRI in cognitive and clinical neuroscience. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01182-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01182-4