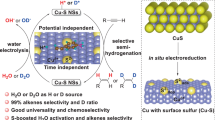
Abstract
The semi-hydrogenation of alkynes to alkenes, especially acetylene to ethylene, is an essential transformation that delivers raw materials and scaffolds for synthetic industries. Electrocatalytic hydrogenation, which is green and mild, provides an alternative strategy to the conventional hydrogenation process, which relies on high temperature, high pressure and flammable H2. This protocol describes an electrocatalytic semi-hydrogenation method to synthesize olefins with water as the hydrogen source under ambient temperature and pressure. Electrocatalytic semi-hydrogenation involves the adsorption and activation of alkynes and the cathodic generation of the active hydrogen (H*) intermediate from water dissociation, followed by the addition of H* to an adsorbed alkyne to yield an alkene. This process is generally assisted by Cu-based electrocatalysts (sulfur-modified Cu and Cu nanoparticles) and commercially available reaction vessels and is performed under a direct-current or constant potential power supply. Here we provide detailed procedures for catalyst design synthesis, alkene electrosynthesis and electrochemical in situ/ex situ spectroscopies for investigating reaction mechanisms. The semi-hydrogenation procedure can be performed within hours; it can also be flexibly adapted to synthetic procedures performed in batch or flow reactors and for various reaction times to meet the adjustable capacity requirements for fine or bulk chemicals. Compared with conventional approaches, the electrocatalytic semi-hydrogenation method eliminates the need for expensive and toxic hydrogenation reagents and conditions with elevated temperature and pressure. Our electrocatalytic semi-hydrogenation strategy has various advantages as a sustainable and alternative method to existing methods, including high alkene selectivity, operational simplicity, substrate universality and easily reproducible functional group compatibility.
Key points
-
This protocol describes an electrocatalytic semi-hydrogenation method to synthesize olefins with water as the hydrogen source under ambient conditions. The procedures for catalyst design synthesis, alkene electrosynthesis and electrochemical in situ/ex situ spectroscopies for investigating the reaction mechanisms are provided in detail.
-
The semi-hydrogenation protocol has the advantages of high alkene selectivity, operational simplicity, substrate universality and easily reproducible functional group compatibility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data discussed in this protocol are available within the figures and the Supplementary Information. Additional data that support the findings of this study can be obtained from the corresponding author upon request.
References
Zhang, L., Zhou, M., Wang, A. & Zhang, T. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms. Chem. Rev. 120, 683–733 (2020).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Shi, R. et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity. Nat. Catal. 4, 565–574 (2021).
Fedorov, A., Liu, H. J., Lo, H. K. & Coperet, C. Silica-supported Cu nanoparticle catalysts for alkyne semihydrogenation: effect of ligands on rates and selectivity. J. Am. Chem. Soc. 138, 16502–16507 (2016).
Tan, Q. et al. Tandem electrocatalytic alkyne semihydrogenation over bicomponent catalysts through hydrogen spillover. Angew. Chem. Int. Ed. 63, e202400483 (2024).
Zhao, X. et al. Thiol treatment creates selective Palladium catalysts for semihydrogenation of internal alkynes. Chem 4, 1080–1091 (2018).
Huang, L., Bao, D., Jiang, Y., Zheng, Y. & Qiao, S.-Z. Electrocatalytic acetylene hydrogenation in concentrated seawater at industrial current densities. Angew. Chem. Int. Ed. 63, e202405943 (2024).
Cao, Y. et al. Adsorption site regulation to guide atomic design of Ni–Ga catalysts for acetylene semi-hydrogenation. Angew. Chem. Int. Ed. 59, 11647–11652 (2020).
Li, B. & Ge, H. Highly selective electrochemical hydrogenation of alkynes: rapid construction of mechanochromic materials. Sci. Adv. 5, eaaw2774 (2019).
Wang, D. S., Chen, Q. A., Lu, S. M. & Zhou, Y. G. Asymmetric hydrogenation of heteroarenes and arenes. Chem. Rev. 112, 2557–2590 (2012).
Liu, C., Chen, F., Zhao, B. H., Wu, Y. & Zhang, B. Electrochemical hydrogenation and oxidation of organic species involving water. Nat. Rev. Chem. 8, 277–293 (2024).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Durin, G. et al. Hydride-free hydrogenation: unraveling the mechanism of electrocatalytic alkyne semihydrogenation by nickel–bipyridine complexes. J. Am. Chem. Soc. 145, 17103–17111 (2023).
Liu, C., Wu, Y., Zhao, B. & Zhang, B. Designed nanomaterials for electrocatalytic organic hydrogenation using water as the hydrogen source. Acc. Chem. Res. 56, 1872–1883 (2023).
Yoshida, J. I., Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Tang, C., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Electrocatalytic refinery for sustainable production of fuels and chemicals. Angew. Chem. Int. Ed. Engl. 60, 19572–19590 (2021).
Bu, J. et al. Selective electrocatalytic semihydrogenation of acetylene impurities for the production of polymer-grade ethylene. Nat. Catal. 4, 557–564 (2021).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. Engl. 57, 5594–5619 (2018).
Wu, Y., Liu, C., Wang, C., Lu, S. & Zhang, B. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd-P cathode. Angew. Chem. Int. Ed. 59, 21170–21175 (2020).
Cummings, S. P., Thanh-Ngoc, L., Fernandez, G. E., Quiambao, L. G. & Stokes, B. J. Tetrahydroxydiboron-mediated palladium-catalyzed transfer hydrogenation and deuteriation of alkenes and alkynes using water as the stoichiometric H or D atom donor. J. Am. Chem. Soc. 138, 6107–6110 (2016).
Harnisch, F. & Morejon, M. C. Hydrogen from water is more than a fuel: hydrogenations and hydrodeoxygenations for a biobased economy. Chem. Rec. 21, 2277–2289 (2021).
Zhang, P. et al. Paired electrocatalytic oxygenation and hydrogenation of organic substrates with water as the oxygen and hydrogen source. Angew. Chem. Int. Ed. Engl. 58, 9155–9159 (2019).
Gao, Y. et al. Field-induced reagent concentration and sulfur adsorption enable efficient electrocatalytic semihydrogenation of alkynes. Sci. Adv. 8, eabm9477 (2022).
Lin, X. et al. Electron divergence of Cuδ− and Pdδ+ in Cu3Pd alloy-based heterojunctions boosts concerted C≡C bond binding and the Volmer step for alkynol semihydrogenation. J. Am. Chem. Soc. 146, 18451–18458 (2024).
Bu, J. et al. Highly selective electrocatalytic alkynol semi-hydrogenation for continuous production of alkenols. Nat. Commun. 14, 1533 (2023).
Meng, L. et al. Alloying and confinement effects on hierarchically nanoporous CuAu for efficient electrocatalytic semi-hydrogenation of terminal alkynes. Nat. Commun. 15, 5999 (2024).
Zhao, Y. et al. Dopant- and surfactant-tuned electrode-electrolyte interface enabling efficient alkynol semi-hydrogenation. J. Am. Chem. Soc. 145, 6516–6525 (2023).
Shi, Y. & Zhang, B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 45, 1529–1541 (2016).
Li, H. et al. σ-Alkynyl adsorption enables electrocatalytic semihydrogenation of terminal alkynes with easy-reducible/passivated groups over amorphous PdSX nanocapsules. J. Am. Chem. Soc. 144, 19456–19465 (2022).
Zhao, B. H. et al. Economically viable electrocatalytic ethylene production with high yield and selectivity. Nat. Sustain. 6, 827–837 (2023).
Song, Z., Yang, R., Liu, X., Zhang, B. & Wu, Y. An organic molecular mimetic metal-free heterogeneous catalyst for electrocatalytic alkyne semihydrogenation. Angew. Chem. Int. Ed. 63, e202410200 (2024).
Chen, F. et al. Ethylene electrosynthesis from low-concentrated acetylene via concave-surface enriched reactant and improved mass transfer. Nat. Commun. 15, 5914 (2024).
Wang, Z. et al. Highly selective acetylene-to-ethylene electroreduction over Cd-decorated Cu catalyst with efficiently inhibited carbon–carbon coupling. Angew. Chem. Int. Ed. 63, e202400122 (2024).
Li, H. et al. Adsorption configuration and H* flux modulation enable electrocatalytic semihydrogenation of alkynes with group tolerance in a palladium membrane reactor. J. Am. Chem. Soc. 147, 17849–17859 (2025).
Wu, Y. et al. Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne semi-hydrogenation with water. Nat. Commun. 12, 3881 (2021).
Akhade, S. A. et al. Electrocatalytic hydrogenation of biomass-derived organics: a review. Chem. Rev. 120, 11370–11419 (2020).
Ling, Y. et al. Selenium vacancy promotes transfer semihydrogenation of alkynes from water electrolysis. ACS Catal. 11, 9471–9478 (2021).
Zhang, L. et al. Deprotonated 2-thiolimidazole serves as a metal-free electrocatalyst for selective acetylene hydrogenation. Nat. Chem. 16, 893–900 (2024).
Wang, S. et al. Highly efficient ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions. Nat. Commun. 12, 7072 (2021).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Zheng, Y., Jiao, Y., Vasileff, A. & Qiao, S. Z. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem. Int. Ed. 57, 7568–7579 (2018).
Li, R. et al. One-pot H/D exchange and low-coordinated iron electrocatalyzed deuteration of nitriles in D2O to α,β-deuterio aryl ethylamines. Nat. Commun. 13, 5951 (2022).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Morales-Guio, C. G., Stern, L. A. & Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 43, 6555–6569 (2014).
Zheng, M. et al. Recent advances in electrocatalytic hydrogenation reactions on copper-based catalysts. Adv. Mater. 36, 2307913 (2024).
Guo, S. et al. Electrocatalytic hydrogenation of quinolines with water over a fluorine-modified cobalt catalyst. Nat. Commun. 13, 5927 (2022).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Czaikowski, M. E., Anferov, S. W., Tascher, A. P. & Anderson, J. S. Electrocatalytic semihydrogenation of terminal alkynes using ligand-based transfer of protons and electrons. J. Am. Chem. Soc. 146, 476–486 (2024).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant no. 2024YFA1510100), the China Postdoctoral Fellowship Program of CPSF (grant no. GZC20241201) and the China Postdoctoral Science Foundation (grant no. 2024M762340) for financial support.
Author information
Authors and Affiliations
Contributions
Y.G., M.H. and B.Z. developed the protocol and co-drafted the manuscript. Y.W., B.-H.Z. and C.L. contributed to the discussion and manuscript modification.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Hanfeng Liang and Shi-Zhang Qiao for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Zhao, B.-H. et al. Nat. Sustain. 6, 827–837 (2023): https://doi.org/10.1038/s41893-023-01084-x
Li, H. et al. J. Am. Chem. Soc. 144, 19456–19465 (2022): https://doi.org/10.1021/jacs.2c07742
Gao, Y. et al. Sci. Adv. 8, eabm9477 (2022): https://doi.org/10.1126/sciadv.abm9477
Wu, Y. et al. Nat. Commun. 12, 3881 (2021): https://doi.org/10.1038/s41467-021-24059-y
Wu, Y. et al. Angew. Chem. Int. Ed. 59, 21170–21175 (2020): https://doi.org/10.1002/anie.202009757
Extended data
Extended Data Fig. 1
Photographs of CF, Cu(OH)2, CuS and CuS.
Extended Data Fig. 2
Photographs of GDL-CP and GDL-CP supported Cu.
Extended Data Fig. 3 Procedure for purifying 4-vinylaniline.
a, The reaction mixture transferred to a separatory funnel. b, Add an additional 15 mL of DCM to the separatory funnel. c, Dry the organic layer with anhydrous Na2SO4. d, Remove EA using a rotary evaporator. e, The obtained product. f, The obtained NMR sample.
Extended Data Fig. 4
The GC with the gas at the flow cell outlet directly injected into it.
Extended Data Fig. 5
The setup for the electrochemical in situ ATR−FTIR measurements.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., He, M., Wu, Y. et al. Electrocatalytic semi-hydrogenation of alkynes using water as the hydrogen source. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01230-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01230-z