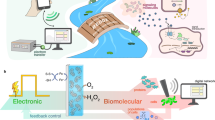
Abstract
Understanding the redox properties and catalytic behavior of proteins is critical for harnessing their functions in biocatalysis and to promote efficient bio-inspired catalysts design. Enzymatic X-ray absorption spectroelectrochemistry (XA-SEC) combines the insights of X-ray absorption spectroscopy with the precision of electrochemical methods to elucidate enzymes’ redox properties and catalytic behavior. Here we describe how to perform enzymatic XA-SEC experiments. The procedure begins with the preparation of the carbon-based working electrode to enhance enzyme immobilization. We exemplify with the efficient immobilization of bilirubin oxidase from Myrothecium verrucaria on the electrode surface, utilizing nanomaterials to enhance biomaterial loading and electron-transfer at the enzyme–electrode interface. Next, we guide researchers through setting up a standard three-electrode electrochemical cell, ensuring proper electrical connections and electrolyte preparation. Our Protocol details the Cu K-edge X-ray absorption spectroscopy measurement procedure at the synchrotron light sources, with in situ electrochemical control. Real-time redox processes are monitored through direct electron transfer analysis, providing valuable thermodynamic and kinetic information. It is important to determine the stability and activity of the analyzed protein under X-ray beam exposure; our approach typically results in stable electrochemical and spectroscopic signals for long experimental runs, showcasing the enzyme’s robust performance and efficient protein immobilization. The method’s ability to correlate XA-SEC data with direct electron transfer and substrate-biding analysis provides a powerful tool for advancing our understanding of enzymatic electrocatalysis and opens new avenues for developing sustainable bioelectrochemical technologies.
Key points
-
X-ray absorption spectroelectrochemistry is crucial to investigating metallic centers within metalloproteins, especially during electrochemical reactions in which redox states change.
-
Performing X-ray absorption spectroelectrochemistry involves multiple details and challenges, either from the protein, the spectroscopic or the electrochemistry sides. As synchrotron sources’ time is scarce, here, we present a Protocol with detailed steps and an extensive troubleshooting section to help researchers acquire useful data within the available time shifts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data from published works by the group are available from the corresponding author upon reasonable request.
Code availability
No code was employed.
References
Andreini, C., Bertini, I., Cavallaro, G., Holliday, G. L. & Thornton, J. M. Metal ions in biological catalysis: from enzyme databases to general principles. J. Biol. Inorg. Chem. 13, 1205–1218 (2008).
Waldron, K. J., Rutherford, J. C., Ford, D. & Robinson, N. J. Metalloproteins and metal sensing. Nature 460, 823–830 (2009).
Zheng, H., Chruszcz, M., Lasota, P., Lebioda, L. & Minor, W. Data mining of metal ion environments present in protein structures. J. Inorg. Biochem. 102, 1765–1776 (2008).
Bowman, S. E. J., Bridwell-Rabb, J. & Drennan, C. L. Metalloprotein crystallography: more than a structure. Acc. Chem. Res. 49, 695–702 (2016).
Dudev, T. & Lim, C. Metal binding affinity and selectivity in metalloproteins: insights from computational studies. Annu. Rev. Biophys. 37, 97–116 (2008).
Stripp, S. T. et al. Second and outer coordination sphere effects in nitrogenase, hydrogenase, formate dehydrogenase, and CO dehydrogenase. Chem. Rev. 122, 11900–11973 (2022).
Timoshenko, J. & Roldan Cuenya, B. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem. Rev. 121, 882–961 (2021).
Sedenho, G. C., Colombo, R. N. P., Iost, R. M., Lima, F. C. D. A. & Crespilho, F. N. Exploring electron transfer: bioinspired, biomimetics, and bioelectrochemical systems for sustainable energy and value-added compound synthesis. Appl. Phys. Rev. 11, 021341 (2024).
Colombo, R. N. P., Sedenho, G. C. & Crespilho, F. N. Challenges in biomaterials science for electrochemical biosensing and bioenergy. Chem. Mater. https://doi.org/10.1021/acs.chemmater.2c02080 (2022).
Butt, J. N., Jeuken, L. J. C., Zhang, H., Burton, J. A. J. & Sutton-Cook, A. L. Protein film electrochemistry. Nat. Rev. Methods Prim. 3, 77 (2023).
Castro, K. P. R., Colombo, R. N. P., Iost, R. M., da Silva, B. G. R. & Crespilho, F. N. Low-dimensionality carbon-based biosensors: the new era of emerging technologies in bioanalytical chemistry. Anal. Bioanal. Chem. https://doi.org/10.1007/s00216-023-04578-x (2023).
Meitzner, G., Gardea-Torresdey, J., Parsons, J., Scott, S. L. & Deguns, E. W. The effect of cryogenic sample cooling on X-ray absorption spectra. Microchem. J. 81, 61–68 (2005).
Macedo, L. J. A., Hassan, A., Sedenho, G. C. & Crespilho, F. N. Assessing electron transfer reactions and catalysis in multicopper oxidases with operando X-ray absorption spectroscopy. Nat. Commun. 11, 316 (2020).
de Souza, J. C. P. et al. In situ and operando techniques for investigating electron transfer in biological systems. ChemElectroChem 8, 431–446 (2021).
Samajdar, R. N. & Bhattacharyya, A. J. Structure-redox response correlation in a few select heme systems using X-ray absorption spectroelectrochemistry. J. Phys. Chem. B 125, 5258–5264 (2021).
Cheaib, K., Maurice, B., Mateo, T., Halime, Z. & Lassalle-Kaiser, B. Time-resolved X-ray absorption spectroelectrochemistry of redox active species in solution. J. Synchrotron Radiat. 26, 1980–1985 (2019).
Cząstka, K., Oughli, A. A., Rüdiger, O. & DeBeer, S. Enzymatic X-ray absorption spectroelectrochemistry. Faraday Discuss. 234, 214–231 (2022).
Macedo, L. J. A. et al. Three-dimensional catalysis and the efficient bioelectrocatalysis beyond surface chemistry. J. Catal. 401, 200–205 (2021).
Fuller, J., Wilson, T. R., Eberhart, M. E. & Alexandrova, A. N. Charge density in enzyme active site as a descriptor of electrostatic preorganization. J. Chem. Inf. Model 59, 2367–2373 (2019).
Sedenho, G. C., Colombo, R. N. P. & Crespilho, F. N. Insights from enzymatic catalysis: a path towards bioinspired high‐performance electrocatalysts. ChemCatChem https://doi.org/10.1002/cctc.202300491 (2023).
Grillo, I. B., Urquiza-Carvalho, G. A., Bachega, J. F. R. & Rocha, G. B. Elucidating enzymatic catalysis using fast quantum chemical Descriptors. J. Chem. Inf. Model 60, 578–591 (2020).
Tolentino, H. C. N. et al. X-ray microscopy developments at Sirius-LNLS: first commissioning experiments at the Carnauba beamline. In Proc. SPIE 11839, X-Ray Nanoimaging: Instruments and Methods V, 1183904 (SPIE, 2021).
Sedenho, G. C. et al. Investigation of water splitting reaction by a multicopper oxidase through X‐ray absorption nanospectroelectrochemistry. Adv. Energy Mater. https://doi.org/10.1002/aenm.202202485 (2022).
Tolentino, H. C. N. et al. The CARNAÚBA X-ray nanospectroscopy beamline at the Sirius-LNLS synchrotron light source: developments, commissioning, and first science at the TARUMÃ station. J. Electron Spectros. Relat. Phenom. 266, 147340 (2023).
Westre, T. E. et al. A multiplet analysis of Fe K-edge 1s → 3d pre-edge features of iron complexes. J. Am. Chem. Soc. 119, 6297–6314 (1997).
Baker, M. L. et al. K- and L-edge X-ray absorption spectroscopy (XAS) and resonant inelastic X-ray scattering (RIXS) determination of differential orbital covalency (DOC) of transition metal sites. Coord. Chem. Rev. 345, 182–208 (2017).
Messias, I. et al. Role of structural and compositional changes of Cu2 O nanocubes in nitrate electroreduction to ammonia. ACS Appl. Energy Mater. 7, 9034–9044 (2024).
Bertella, F., Lopes, C. W., Benvenutti, E. V. & de Souza, M. O. Unveiling the stability of mixed Zn/Co-ZIFs as catalysts for CO2 fixation into cyclic carbonates. Catal. Today 445, 115096 (2025).
Fritzen, D. L. et al. From synthesis to fabrication: engineering thin translucent films with green persistent luminescent nanoparticles. Opt. Mater. 20, 100271 (2023).
Fang, L., Seifert, S., Winans, R. E. & Li, T. Understanding synthesis and structural variation of nanomaterials through in situ/operando XAS and SAXS. Small 18, e2106017 (2022).
Alves, F. B. et al. Facilitating seed iron uptake through amine-epoxide microgels: a novel approach to enhance cucumber (Cucumis sativus) germination. J. Agric. Food Chem. 72, 14570–14580 (2024).
Blank, M. A. et al. Structural models of the [Fe4S4] clusters of homologous nitrogenase Fe proteins. Inorg. Chem. 50, 7123–7128 (2011).
Solomon, E. I., Szilagyi, R. K., DeBeer George, S. & Basumallick, L. Electronic structures of metal sites in proteins and models: contributions to function in blue copper proteins. Chem. Rev. 104, 419–458 (2004).
Feiters, M. C. X-ray absorption spectroscopic studies of metal coordination in zinc and copper proteins. Comments Inorg. Chem. 11, 131–174 (1990).
Bobyr, E. et al. High-resolution analysis of Zn2+ coordination in the alkaline phosphatase superfamily by EXAFS and X-ray crystallography. J. Mol. Biol. 415, 102–117 (2012).
Penner-Hahn, J. E. et al. Structural characterization of the manganese sites in the photosynthetic oxygen-evolving complex using X-ray absorption spectroscopy. J. Am. Chem. Soc. 112, 2549–2557 (1990).
Wu, A. J., Penner-Hahn, J. E. & Pecoraro, V. L. Structural, spectroscopic, and reactivity models for the manganese catalases. Chem. Rev. 104, 903–938 (2004).
de Barros, H. R., Miguel, V. M., Colombo, R. N. P., da Silva, R. T. P. & de Torresi, S. I. C. in Advances in Bioelectrochemistry (ed. Crespilho, F. N.) Vol. 5, 37–83 (Springer International Publishing, 2023).
Le Ru, E. C. & Etchegoin, P. G. Quantifying SERS enhancements. MRS Bull. 38, 631–640 (2013).
Colombo, R. N. P., Moreira, R. V., de Faria, D. L. A. & Córdoba de Torresi, S. I. Controlling gold electrodeposition on porous polymeric templates produced by the breath‐figure method: fabrication of SERS‐active surfaces. ChemPlusChem 84, 1052–1059 (2019).
de Souza, J. C. P., Silva, W. O., Lima, F. H. B. & Crespilho, F. N. Enzyme activity evaluation by differential electrochemical mass spectrometry. Chem. Commun. 53, 8400–8402 (2017).
Iost, R. M., Radhakrishnan, V., Nascimento, S. Q., Lima, F. H. B. & Crespilho, F. N. Hydrogen bioelectrogeneration with PH-resilient and oxygen-tolerant cobalt apoenzyme-saccharide. Chem. Commun. https://doi.org/10.1039/D3CC06185J (2024).
Mendes, G. R., de Modenez, I. A., Cagnani, G. R., Colombo, R. N. P. & Crespilho, F. N. Exploring enzymatic conformational dynamics at surfaces through μ-FTIR spectromicroscopy. Anal. Chem. https://doi.org/10.1021/acs.analchem.3c00872 (2023).
Maghraby, Y. R., El-Shabasy, R. M., Ibrahim, A. H. & Azzazy, H. M. E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 8, 5184–5196 (2023).
Datta, S., Christena, L. R. & Rajaram, Y. R. S. Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3, 1–9 (2013).
Jesionowski, T., Zdarta, J. & Krajewska, B. Enzyme immobilization by adsorption: a review. Adsorption 20, 801–821 (2014).
Masalova, O., Kulikouskaya, V., Shutava, T. & Agabekov, V. Alginate and chitosan gel nanoparticles for efficient protein entrapment. Phys. Procedia 40, 69–75 (2013).
Sabaté del Río, J., Henry, O. Y. F., Jolly, P. & Ingber, D. E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 14, 1143–1149 (2019).
Colombo, R. N. P., Nascimento, S. Q. & Crespilho, F. N. Conductance channels in a single-entity enzyme. J. Phys. Chem. Lett. https://doi.org/10.1021/acs.jpclett.4c01796 (2024).
Shein, J. B., Lai, L. M. H., Eggers, P. K., Paddon-Row, M. N. & Gooding, J. J. Formation of efficient electron transfer pathways by adsorbing gold nanoparticles to self-assembled monolayer modified electrodes. Langmuir 25, 11121–11128 (2009).
Holzinger, M., Goff, A., Le & Cosnier, S. Nanomaterials for biosensing applications: a review. Front. Chem. https://doi.org/10.3389/fchem.2014.00063 (2014).
Fogel, R. & Limson, J. L. Probing fundamental film parameters of immobilized enzymes—towards enhanced biosensor performance. Part I—QCM-D mass and rheological measurements. Enzym. Microb. Technol. 49, 146–152 (2011).
Bartlett, P. N. & Al-Lolage, F. A. There is no evidence to support literature claims of direct electron transfer (DET) for native glucose oxidase (GOx) at carbon nanotubes or graphene. J. Electroanal. Chem. https://doi.org/10.1016/j.jelechem.2017.06.021 (2017).
Vicente, R. A. et al. Development of electrochemical cells and their application for spatially resolved analysis using a multitechnique approach: from conventional experiments to X-ray nanoprobe beamlines. Anal. Chem. 95, 16144–16152 (2023).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2018).
Marken, F., Neudeck, A. & Bond, A. M. in Electroanalytical Methods (ed. Scholz, F.) 57–106 (Springer, 2010).
Léger, C. et al. Enzyme electrokinetics: using protein film voltammetry to investigate redox enzymes and their mechanisms. Biochemistry 42, 8653–8662 (2003).
Brewer, P. J., Leese, R. J. & Brown, R. J. C. An improved approach for fabricating Ag/AgCl reference electrodes. Electrochim. Acta 71, 252–257 (2012).
Yang, H., Yang, S., Kong, J., Dong, A. & Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 10, 382–396 (2015).
Micsonai, A. et al. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl Acad. Sci. USA 112, E3095–E3103 (2015).
Siegbahn, P. E. M. Theoretical study of O2 reduction and water oxidation in multicopper oxidases. J. Phys. Chem. A 124, 5849–5855 (2020).
Gonçales, V. R. et al. Three-dimensional graphene/carbon nanotubes hybrid composites for exploring interaction between glucose oxidase and carbon based electrodes. J. Electroanal. Chem. 775, 235–242 (2016).
Hassan, A., Colombo, R. N. P., Iost, R. M. & Crespilho, F. N. Amplifying sensing performance through gold micropatterns-induced modulation of graphene’s vertical electron transfer. Electrochim. Acta 466, 143069 (2023).
Fatima, S. et al. Engineering a conformationally switchable artificial metalloprotein. J. Am. Chem. Soc. 144, 21606–21616 (2022).
Acknowledgements
We thank São Paulo Research Foundation - FAPESP for the financial support, with grant nos. 2021/05665-7 (R.N.P.C.), 2018/22214-6, 2022/09164-5, 2023/01529-7 (F.N.C.) and 2020/04796-8 (G.C.S.). This research used resources from the Brazilian Synchrotron Light Laboratory (LNLS), part of the Brazilian Center for Research in Energy and Materials (CNPEM), a private nonprofit organization under the supervision of the Brazilian Ministry for Science, Technology and Innovations (MCTI). The Carnauba beamline staff are acknowledged for their assistance during the experiments.
Author information
Authors and Affiliations
Contributions
R.N.P.C., G.C.S., I.T.N. and F.N.C. contributed to writing, editing and formatting. F.N.C. provided the initial draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Elisabeth Lojou, Soumalya Sinha and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Sedenho, G. C. et al. Adv. Energy Matter. 12, 2202485 (2022): https://doi.org/10.1002/aenm.202202485
Macedo, L. J. A. et al. Nat. Commun. 11, 316 (2020): https://doi.org/10.1038/s41467-019-14210-1
Sedenho, G. C. et al. Appl. Phys. Rev. 11, 021341 (2024): https://doi.org/10.1063/5.0204996
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Colombo, R.N.P., Sedenho, G.C., Neckel, I.T. et al. Enzymatic X-ray absorption spectroelectrochemistry. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01254-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01254-5