Abstract
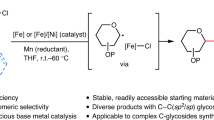
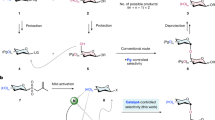
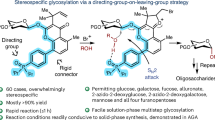
Complex carbohydrates are essential to life processes, but it is challenging to isolate these molecules from natural sources in high homogeneity. Therefore, complex-glycan synthesis becomes critical to improving our understanding of their important functions. Due to their complexity, synthesis is still difficult for nonexperts. One of the key challenges is to search for general solutions for highly 1,2-cis-selective glycosylation, which will directly assemble 1,2-cis-2-aminoglycosides that are incorporated in numerous biologically important complex glycans and glycoconjugates. Here we describe an iron-catalyzed, chemical glycosylation method for rapid assembly of 1,2-cis-aminoglycosidic linkages. The iron catalyst is commercially available, and the bench-stable supporting ligand and amination reagents are easily prepared from abundant, readily available starting materials. This catalytic, exclusively 1,2-cis-selective glycosylation is effective for a broad range of glycosyl donors and acceptors, and it can be operated in a continuous fashion and scaled up to the multigram scale. The reactivity of this glycosylation is tunable for both electron-rich and electron-deficient substrates by modulating amination reagents. The glycosylation proceeds through a unique mechanism in which the iron catalyst activates a glycosyl acceptor and an oxidant when it facilitates the cooperative atom transfer of both moieties to a glycosyl donor in an exclusively cis-selective manner. This glycosylation protocol takes several hours to operate. It complements the existing 1,2-cis-selective glycosylation methods and effectively addresses the challenge of achieving both generality and high stereoselectivity in the 1,2-cis-selective aminoglycosylation.
Key points
-
The synthesis of complex carbohydrates for research studies is difficult. A major challenge is that most methods are not generalizable, because small structural changes in the starting materials can have a large impact on the stereoselectivity of the reactions.
-
This Protocol describes a robust, iron-catalyzed glycosylation method for rapid assembly of 1,2-cis-aminoglycosidic linkages within several hours. It is effective for a broad range of substrates, and it can be operated in a continuous fashion and scaled up to the multigram scale.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Experimental procedures and characterization data for all described compounds and selected NMR spectra are included in the Supporting Information of this Protocol and/or the Supplementary Information, which is available free of charge on https://doi.org/10.1021/jacs.4c15084.
References
Danishefsky, S. J. & Bilodeau, M. T. Glycals in organic synthesis: the evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew. Chem. Int. Ed. 35, 1380–1419 (1996).
Boltje, T. J., Buskas, T. & Boons, G.-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 1, 611–622 (2009).
Zhu, X. & Schmidt, R. R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. 48, 1900–1934 (2009).
Danishefsky, S. J., Shue, Y.-K., Chang, M. N. & Wong, C.-H. Development of globo-H cancer vaccine. Acc. Chem. Res. 48, 643–652 (2015).
Park, Y. et al. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science 355, 162–166 (2017).
Bennett, C.S. Selective Glycosylations: Synthetic Methods and Catalysts (Wiley-VCH, 2017).
Yu, B. Gold(I)-catalyzed glycosylation with glycosyl O-alkynylbenzoates as donors. Acc. Chem. Res. 51, 507–516 (2018).
Levi, S. M. & Jacobsen, E. N. in Organic Reactions Vol. 100, 801–852 (John Wiley and Sons, 2019).
Nigudkar, S. S. & Demchenko, A. V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 6, 2687–2704 (2015).
Xu, Y. et al. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science 334, 498–501 (2011).
Petitou, M. & van Boeckel, C. A. A. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Ed. 43, 3118–3133 (2004).
Pratt, M. R. & Bertozzi, C. R. Synthetic glycopeptides and glycoproteins as tools for biology. Chem. Soc. Rev. 34, 58–68 (2005).
Griffith, D. A. & Danishefsky, S. J. Sulfonamidoglycosylation of glycals. A route to oligosaccharides with 2-aminohexose subunits. J. Am. Chem. Soc. 112, 5811–5819 (1990).
Fitzsimmons, B. J., Leblanc, Y. & Rokach, J. [4 + 2] Cycloaddition of azodicarboxylate and glycals: a novel and simple method for the preparation of 2-amino-2-deoxy carbohydrates. J. Am. Chem. Soc. 109, 285–286 (1987).
Du Bois, J., Tomooka, C. S., Hong, J. & Carreira, E. M. Novel, stereoselective synthesis of 2-amino saccharides. J. Am. Chem. Soc. 119, 3179–3180 (1997).
Di Bussolo, V., Liu, J., Huffman, J. L. G. & Gin, D. Y. Acetamidoglycosylation with glycal donors: a one-pot glycosidic coupling with direct installation of the natural C(2)-N-acetylamino functionality. Angew. Chem. Int. Ed. 39, 204–207 (2000).
Kan, C. et al. Photo amidoglycosylation of an allal azidoformate. Synthesis of β-2-amido allopyranosides. Org. Lett. 3, 381–384 (2001).
Gege, C., Oscarson, S. & Schmidt, R. R. Synthesis of fluorescence labeled Sialyl LewisX glycosphingolipids. Tetrahedron Lett. 42, 377–380 (2001).
Shang, W. et al. Nitrogen-centered radical-mediated cascade amidoglycosylation of glycals. Org. Lett. 23, 1222–1227 (2021).
Xu, Y. & Montgomery, J. Synthesis of 2-amino-2-deoxy sugars via boron-catalyzed coupling of glycosyl fluorides and silyl ether acceptors. Org. Lett. 26, 7474–7478 (2024).
Dulaney, S. B. & Huang, X. Strategies in synthesis of heparin/heparan sulfate oligosaccharides: 2000–present. Adv. Carbohydr. Chem. Biochem. 80, 121–164 (2021).
Winterfeld, G. A. & Schmidt, R. R. Nitroglycal concatenation: a broadly applicable and efficient approach to the synthesis of complex O-glycans. Angew. Chem. Int. Ed. 40, 2654–2657 (2001).
Benakli, K., Zha, C. & Kerns, R. J. Oxazolidinone protected 2-amino-2-deoxy-d-glucose derivatives as versatile intermediates in stereoselective oligosaccharide synthesis and the formation of α-linked glycosides. J. Am. Chem. Soc. 123, 9461–9462 (2001).
Orgueira, H. A., Bartolozzi, A., Schell, P. & Seeberger, P. H. Conformational locking of the glycosyl acceptor for stereocontrol in the key step in the synthesis of heparin. Angew. Chem. Int. Ed. 41, 2128–2131 (2002).
Xue, J. & Guo, Z. Convergent synthesis of an inner core GPI of sperm CD52. Bioorg. Med. Chem. Lett. 12, 2015–2018 (2002).
Manabe, S., Ishii, K. & Ito, Y. N-benzyl-2,3-oxazolidinone as a glycosyl donor for selective α-glycosylation and one-pot oligosaccharide synthesis involving 1,2-cis-glycosylation. J. Am. Chem. Soc. 128, 10666–10667 (2006).
Park, J., Kawatkar, S., Kim, J.-H. & Boons, G.-J. Stereoselective glycosylations of 2-azido-2-deoxy-glucosides using intermediate sulfonium ions. Org. Lett. 9, 1959–1962 (2007).
Mensah, E. A. & Nguyen, H. M. Nickel-catalyzed stereoselective formation of α-2-deoxy-2-amino glycosides. J. Am. Chem. Soc. 131, 8778–8780 (2009).
Mensah, E. A., Yu, F. & Nguyen, H. M. Nickel-catalyzed stereoselective glycosylation with C(2)-N-substituted benzylidene d-glucosamine and galactosamine trichloroacetimidates for the formation of 1,2-cis-2-amino glycosides. Applications to the synthesis of heparin disaccharides, GPI anchor pseudodisaccharides, and α-GalNAc. J. Am. Chem. Soc. 132, 14288–14302 (2010).
Yoshida, K. et al. Chemical synthesis of syndecan-3 glycopeptides bearing two heparan sulfate glycan chains. Angew. Chem. Int. Ed. 53, 9051–9058 (2014).
Medina, S. et al. Stereoselective glycosylation of 2-nitrogalactals catalyzed by a bifunctional organocatalyst. Org. Lett. 18, 4222–4225 (2016).
Codeé, J. D. C., Wang, L., Zhang, Y., Overkleeft, H. S. & van der Marel, G. A. Reagent controlled glycosylations for the assembly of well-defined pel oligosaccharides. J. Org. Chem. 85, 15872–15884 (2020).
Pal, K. B., Guo, A., Das, M., Báti, G. & Liu, X. W. Superbase-catalyzed stereo- and regioselective glycosylation with 2-nitroglycals: facile access to 2-amino-2-deoxy-O-glycosides. ACS Catal. 10, 6707–6715 (2020).
Zhang, Y., Ma, X. & Zhang, L. Highly stereoselective synthesis of 2-azido-2-deoxyglycosides via gold-catalyzed SN2 glycosylation. CCS Chem. 5, 2799–2807 (2023).
Liu, G.-S., Zhang, Y.-Q., Yuan, Y.-A. & Xu, H. Iron(II)-catalyzed intramolecular aminohydroxylation of olefins with functionalized hydroxylamines. J. Am. Chem. Soc. 135, 3343–3346 (2013).
Lu, D.-F., Zhu, C.-L., Jia, Z.-X. & Xu, H. Iron(II)-catalyzed intermolecular amino-oxygenation of olefins through the n–o bond cleavage of functionalized hydroxylamines. J. Am. Chem. Soc. 136, 13186–13189 (2014).
Lu, D.-F., Zhu, C.-L., Sears, J. D. & Xu, H. Iron(II)-catalyzed intermolecular aminofluorination of unfunctionalized olefins using fluoride ion. J. Am. Chem. Soc. 138, 11360–11367 (2016).
Li, H. et al. Stereoselective glycosylation for 1,2-cis-aminoglycoside assembly by cooperative atom transfer catalysis. J. Am. Chem. Soc. 146, 33316–33323 (2024).
Yin, L., Zhang, D., Jiang, Z. & Xu, H. Stereoselective multigram-scale Tn antigen synthesis via the iron-catalyzed glycal 1,2-cis-aminoglycosylation. Org. Lett. 27, 5515–5520 (2025).
Yin, L. et al. Iron-catalyzed glycal cis-aminoacyloxylation for 2-amino saccharide synthesis. Tetrahedron Lett. 167, 155678 (2025).
Xu, H. et al. Iron-catalyzed stereoselective nitrogen atom transfer for 1,2-cis-selective glycosylation. Synlett https://doi.org/10.1055/a-2654-5609 (2025).
Seeberger, P. H. The logic of automated glycan assembly. Acc. Chem. Res. 48, 1450–1463 (2015).
Halcomb, R. L. & Danishefsky, S. J. On the direct epoxidation of glycals: application of a reiterative strategy for the synthesis of b-linked oligosaccharides. J. Am. Chem. Soc. 111, 6661–6666 (1989).
Danishefsky, S. J., McClure, K. F., Randolph, J. T. & Ruggeri, R. B. A strategy for the solid-phase synthesis of oligosaccharides. Science 260, 1307–1309 (1993).
Acknowledgements
This research was supported by the National Institutes of Health (grant no. GM134926). We thank NIH Shared Instrumentation grant S10OD034395 (NMR) and NSF MRI program 1919565 (Single Crystal X-ray diffractometer) for the instrument support.
Author information
Authors and Affiliations
Contributions
Z.J. designed and performed the experiments and cowrote the paper. D.Z. designed and performed the experiments and cowrote the paper. P.W. synthesized catalyst 1 and performed the experiments. L.Y. performed the experiments. H.X. designed and supervised the experiments, analyzed data and cowrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests. The subject matter described in this article is included in patent applications filed by Brandeis University.
Peer review
Peer review information
Nature Protocols thanks Ram Sagar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Li, H. et al. J. Am. Chem. Soc. 146, 33316–33323 (2024): https://doi.org/10.1021/jacs.4c15084
Yin, L., Zhang, D., Jiang, Z. & Xu, H. Org. Lett. 27, 5515–5520 (2025): https://doi.org/10.1021/acs.orglett.5c01560
Lu, D.-F., Zhu, C.-L., Jia, Z.-X. & Xu, H. J. Am. Chem. Soc. 136, 13186–13189 (2014): https://doi.org/10.1021/ja508057u
Liu, G.-S., Zhang, Y.-Q., Yuan, Y.-A. & Xu, H. J. Am. Chem. Soc. 135, 3343–3346 (2013): https://pubs.acs.org/doi/10.1021/ja311923z?ref=recommended
Yin, L. et al. Tetrahedron Lett. 167, 155678 (2025): https://www.sciencedirect.com/science/article/abs/pii/S0040403925002278?via%3Dihub
Supplementary information
Supplementary Information
A. General information. B. Protocols for synthesis of substrates and amination reagents 3, 9, 11, 14, 16 and 17c. C. A procedure for selective N-Boc deprotection. D. Procedures for rapid post-glycosylation deprotection to afford Tn antigen. E. Procedures for the iron-catalyzed reiterative glycal 1,2-cis-aminoglycosylation. F. References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Z., Zhang, D., Wang, P. et al. Iron-catalyzed stereoselective glycosylation for 1,2-cis-aminoglycoside assembly. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01263-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41596-025-01263-4