Abstract
Rising temperatures represent a significant threat to the survival of ectothermic animals. As such, upper thermal limits represent an important trait to assess the vulnerability of ectotherms to changing temperatures. For instance, one may use upper thermal limits to estimate current and future thermal safety margins (i.e., the proximity of upper thermal limits to experienced temperatures), use this trait together with other physiological traits in species distribution models, or investigate the plasticity and evolvability of these limits for buffering the impacts of changing temperatures. While datasets on thermal tolerance limits have been previously compiled, they sometimes report single estimates for a given species, do not present measures of data dispersion, and are biased towards certain parts of the globe. To overcome these limitations, we systematically searched the literature in seven languages to produce the most comprehensive dataset to date on amphibian upper thermal limits, spanning 3,095 estimates across 616 species. This resource will represent a useful tool to evaluate the vulnerability of amphibians, and ectotherms more generally, to changing temperatures.
Measurement(s) | CTmax • Critical thermal maximum • LT50 • Median lethal temperature • Thermal tolerance • Thermal limits |
Technology Type(s) | experimental |
Factor Type(s) | Location • Conservation status • Environmental temperature • Laboratory temperatures • Body size • Ontogeny • Methodological variation |
Sample Characteristic - Organism | Amphibians • Caudata • Amphibia • Frogs • Salamanders • Newts |
Sample Characteristic - Environment | natural environment • laboratory environment |
Sample Characteristic - Location | Global |
Similar content being viewed by others
Background & Summary
The Earth is warming at an unprecedented rate, with extreme heat waves being predicted to increase in both frequency and intensity1. Understanding how biodiversity will respond to drastic changes in temperatures is thus paramount for their continued survival. One possibility would be to use the thermal tolerance of species to assess changes in species geographic ranges and extinction risk2,3. While the extent to which animals tolerate temperatures can be assessed in various ways, the estimation of upper thermal limits—the temperature at which animals lose chances of survival4,5,6—forms a crucial piece of information to project the impacts of ongoing climate change. Notably, experimentally derived upper thermal limits are great proxies of the fundamental thermal niche of animals7 and have enabled important findings in ecophysiology. For instance, large empirical studies and syntheses have demonstrated that most ectotherms are intolerant to extreme temperatures8, that species inhabiting tropical and marine environments are particularly vulnerable2,9,10, that early-life stages represent the weakest link in the life cycle11,12,13,14, and that the plasticity14,15,16,17,18 and evolutionary potential of upper thermal limits is insufficient to compensate for rising temperatures19,20,21,22,23.
While previous data compilations were rigorous2,15,16,17,18,24,25,26, there are four major areas for improvement to increase their usability and reliability. First, data compilations require a representative and comprehensive sample of the literature to best inform macrophysiological projections. For example, data imputation procedures assume specific missing data patterns (e.g., data missing at random27). Thus, any biases in the data may compromise the validity of imputation procedures28. While compiling a comprehensive sample of the literature is challenging, one can reach comprehensiveness by systematically reviewing both grey and traditional academic literature from numerous databases29. Second, the geographical coverage of most physiological databases is relatively poor24,30,31. One way to minimise this bias is to conduct the literature search in non-English languages because a non-negligible amount of non-English-language literature still exists in countries where English is not widely spoken30,32. Third, some datasets on thermal tolerance have limited their scope to one comparable estimate per species24,25,26. However, quantifying within-species variation is of particular interest to comparative physiologists33,34. In fact, accumulating evidence support that thermal tolerance varies substantially within the same species due to plasticity and local adaptation e.g.11,14,17,35,36,. Notably, ectotherms raised at higher temperatures tend to tolerate higher temperatures14,15,16,17,18. The incorporation of plastic responses, among other forms of intraspecific variation, is thus incredibly important to accurately inform the future of animals on this planet. Fourth, accounting for measures of statistical dispersion (e.g., standard deviation) is vital to weight findings based on their precision because thermal limit estimates are often underpowered34,37,38. Performing unweighted analyses puts an unnecessarily large emphasis on low-powered estimates. Moreover, the unavailability of measures of dispersion in datasets constrains the usability of data for meta-analyses as they are often needed to calculate effect sizes and their associated sampling variance (e.g., log response ratio).
Here, we bridge the gaps in previous databases by systematically reviewing upper thermal limits of amphibian species. We focused on amphibians because 41% of the assessed species are threatened39, and temperature is an important driver of their extinction40,41,42. We compiled a comprehensive and representative sample of the literature through scoping various databases and assessing the literature in seven different languages to increase the representation of data from traditionally under-represented (and non-English speaking) countries30. We also included various forms of intraspecific variation (e.g., different acclimation temperatures, different life stages) to provide a more comprehensive and flexible picture of how amphibians cope with extreme temperatures. Finally, our data contain measures of dispersion for most (75%) estimates, which will facilitate the inclusion of these data in meta-analyses.
Methods
Literature searches
We aimed to obtain a comprehensive and representative sample of the published and unpublished experimental literature assessing the upper thermal limits of amphibian species. We accessed Scopus, ISI Web of Science (core collection), Lens, and ProQuest (dissertation & theses) on 2021/05/31 using The University of New South Wales’ institution subscriptions and did not apply a time span limit. We also obtained non-English language literature by conducting additional searches in Google Scholar in traditional Chinese, simplified Chinese, French, Japanese, Portuguese, and Spanish. We chose these languages to maximise the discovery of findings where amphibian species diversity is the highest43. We did not assess additional languages due to time constraints and excluded languages for which we found limited evidence during pilot searches. For instance, this included common Southeast Asian languages such as Malay, Bahasa Indonesia, and Vietnamese.
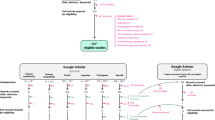
English search strings were adapted to the structure of each database (Supplementary Table S1), and we complemented our searches with backward searches, where we collected studies cited in previous compilations of ectotherm or amphibian upper thermal limits2,15,16,17,24,25. Our database and backward searches retrieved 1672 unique documents that were screened by PPottier for title, abstract and keyword content in Rayyan QCRI44. We used this software to highlight important keywords and filter publications based on our inclusion criteria. A total of 271 documents were then assessed for eligibility by PPottier (81.5%) and YY (18.5%) and 177 studies were found eligible.
Translated searches were performed in Google Scholar because other databases provide poor indexing of studies published in languages other than English. Indeed, respectively 95.37% and 92.64% of the documents indexed in Scopus and Web of Science are in English45. Although Google Scholar suffers from reproducibility issues46, it is recommended as a complementary database to systematic reviews47,48. A suite of keywords was translated by native speakers: “thermal tolerance”, “critical temperature”, “thermal limit”, “lethal temperature”, “amphibian”, “frog”, “toad”, “salamander”, “newt”, “tadpole”. Each keyword (or combination of keywords) was translated in its singular and/or plural form, where applicable. Because Google Scholar’s search strings have a limitation of 256 characters, each keyword (or combination of keywords) was sequentially assessed in its singular or plural form, and the search returning the greatest hit rate was selected. All Google scholar searches had the form (“thermal tolerance” OR synonyms) AND (amphibian OR synonyms). For each language, we also performed a search where we replaced “thermal tolerance” by “CTmax”, a keyword used in various languages (Supplementary Table S1). Bibliographic records were extracted from Google Scholar using Publish or Perish49. We also found two additional studies in traditional Chinese50,51 during pilot searches in Google (benchmarking sensu29). NR (50%) and JOV (50%) performed the screening of articles in Spanish, while YY, RRYO, PPottier, SN, and PPollo screened documents in traditional Chinese, simplified Chinese, French, Japanese, and Portuguese, respectively. Twenty eligible studies in languages other than English were found following those searches. Moreover, we found 11 eligible studies published in English that were not retrieved by the English searches we performed (Fig. 1). Eventually, 14.6% of the included studies were retrieved through searches in languages other than English.
PRISMA flowchart. Highlighted are the different databases used, the number of studies included at each stage of the screening, and the reasons for excluding studies. *The five studies found during non-systematic searches include two studies found during pilot searches in Google, and three studies mistakenly excluded during the screening, but included in a previous synthesis by Rohr and colleagues16.
Eligibility criteria
We focused on experimental studies that measured the upper thermal limit of wild and laboratory amphibians. We selected studies based on three main criteria. First, we only considered data on juveniles and adults. We excluded studies on embryonic traits because methods used to quantify their upper thermal limits tend to differ from those used for juveniles and adults. Second, we only included studies using the most reported measures of upper thermal limits, which are analogous to the fundamental thermal niche animals can exploit. Specifically, we included studies measuring the critical thermal maximum (CTmax) or the median lethal temperature (LT50) of amphibians. CTmax represents a measure of upper thermal limit where the temperature is increased progressively until an endpoint is reached (e.g., loss of righting response5). Conversely, LT50 is assessed by subjecting animals to constant temperatures and measuring their survival after a given period4. The temperature lethal for 50% of the animals (LT50) is then interpolated from the survival data at different temperatures. We also considered studies reporting the time to death at different static temperatures because these data can be converted to CTmax estimates52. Because CTmax occurs before death and the conversion between static and dynamic CTmax relies on certain assumptions, the accuracy of our conversions needs statistical validation before inclusion in statistical analyses. We chose to focus on these proxies to maximise the comparability of estimates across species and therefore, the usability of the database. Third, because upper thermal limits are intrinsically plastic14,15,16,17, we only considered studies reporting the temperature at which animals were maintained in the laboratory (i.e., the temperature of acclimation), the temperature of the environment from which animals were captured (i.e., the temperature of acclimatization), or the geographical coordinates and dates of capture (which may allow the estimation of acclimatization temperatures). Decision trees and inclusion criteria are presented in Supplementary Figures S1-2 and Tables S3-4. Search methods and reasons for excluding studies are summarised in a PRISMA flowchart (Fig. 1). Ultimately, we retrieved 213 relevant publications42,50,51,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262.
Data extraction
We extracted the mean upper thermal limit of amphibians along with their associated sample sizes and measures of dispersion (i.e., standard deviation or standard error). Data extractions were performed by PPottier (50.0%), HYL (8.9%), RRYO (7.5%), ANRV (9.4%), PPollo (8.9%), JOV (8.9%) and YY (6.5%). Data from text and tables were directly extracted, while data presented in figures were digitised using the metaDigitise package263 (v. 1.0.1) in R264 (v. 4.1.3). We also calculated means, standard deviations, and sample sizes from the raw data, when available. When data were presented from different sources (e.g., table and figure), we favoured the source having the highest resolution (e.g., extract the data for each sex instead of the pooled data for both sexes). For studies only reporting the survival time at different temperatures, we converted these data to CTmax using the thermal tolerance landscape framework6,52. We performed a linear regression of the logarithm of the time to the death against the test temperatures, and interpolated the temperature amphibians could tolerate for 1 hour as a proxy for CTmax (following the recommendations of52). For the 85 studies included in the synthesis by Rohr and colleagues16, we extracted the data presented in this synthesis, and we completed missing cases using the original study. However, we re-extracted the data from tables, figures and/or raw data when data could be extracted at a finer resolution.
We also extracted other variables to account for potential sources of heterogeneity and non-independence in the data. Notably, we assigned unique identifiers for each estimate, study, species, population, and cohort to encourage quantifying the variance explained by those variables (see34). We included all sources of within-species variation presented in the original studies. This includes, for instance, data from experimental groups exposed to different acclimation temperatures, different life stages, different populations, or different endpoints used to infer upper thermal limits. The full list of extracted variables is described in Supplementary Table S2.
Species names and taxonomy were standardized according to the most comprehensive phylogenetic tree for amphibians to date265, which mostly aligns with AmphibiaWeb266. We also included data from species not described at the species level (e.g., Noblella sp.) and species not indexed in AmphibiaWeb266 or presented in265. We believe that this database will be updated, and therefore the taxonomic information may change, and be useful to future users. As our study focused on naturally occurring species, we did not include data from experimentally bred hybrid species.
Data were organised in a ‘stacked’ format (following the recommendations of267), to facilitate their use in comparative studies. Note that these data can be converted to an ‘effect size’ format for meta-analysis.
Data curation
Because the data we extracted contains various sources of within-species variability, estimates may not be directly comparable for most comparative analyses. Therefore, we provided a curated version of the database, which excludes data having procedural concerns (see Supplementary Table S5); animals exposed to toxicants, hormones, or high levels of UV radiations; extinct species; and species not described at the species level. This subset of the data (197 studies, 587 species) is suitable for answering most questions in ecophysiology. A script detailing each step of the data curation is available via Zenodo268.
Data Records
This database includes upper thermal limits of 616 amphibian species (~7% of all described species) across 37 families, with a broad geographical and phylogenetic coverage (Figs. 2–4). There are three key strengths of this database. First, it captures the substantial within-species variation, wherein 5.02 ± 12.6 (mean ± SD) upper thermal limit estimates have been extracted for each species on average (range: 1–163 estimates per species). Second, it formally includes data from non-English-language publications. In fact, 27 studies included in our synthesis were published in languages other than English, which represented 17 estimates taken from publications in Portuguese (9 species, 2 studies), 61 estimates from Spanish (37 species, 8 studies), 53 estimates from simplified Chinese (12 species, 10 studies) and 233 estimates from traditional Chinese publications (21 species, 6 studies). Third, our systematic review can easily be reproduced, updated, and expanded to a wider range of species, including other ectothermic taxa.
Distribution of estimates and mean upper thermal limits across the phylogeny of species included in the database. (A) adults. (B) larvae and juveniles. The number of estimates compiled per species (histograms) is presented on a log2(x + 1) scale. Phylogeny is based on the consensus of 10,000 fully-sampled trees from the posterior distribution, as described in265. a. Rana pipiens © Brian Gratwicke, b. Plethodon cylindraceus © Brian Gratwicke, c. Notophthalmus viridescens © Brian Gratwicke, d. Dendropsophus ebraccatus © Brian Gratwicke, e. Pleurodema thaul © Richard Sage, f. Craugastor longirostris, g. Limnodynastes peronii © Evan Pickett, h. Microhyla heymonsi, i. Ambystoma mexicanum © Tim Flach, j. Hyla versicolor © Michael F. Benard, k. Engystomops pustulosus © Daniel J. Paluh. l. Anaxyrus americanus © Brian Gratwicke.
Distribution of estimates across the phylogeny of nearly all extant amphibians. The upper thermal limits of amphibian species included in our database are mapped on the phylogeny of nearly all extant species (7,238 species). Family names are presented in the outer circle, which was adapted from265. Phylogeny is based on the consensus of 10,000 fully-sampled trees from the posterior distribution, as described in265.
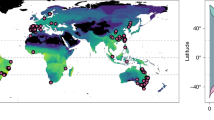
Geographical locations at which experimental data were collected. Points denote which order of amphibians were assayed (point filling), at which life stage (point border) and in what language were the findings published (point shape). Note that geographical coordinates were missing for 659 (21.3%) of the estimates, notably when animals were raised in the laboratory for numerous generations.
Our database is hosted in Github (https://github.com/p-pottier/Amphibian_upper_thermal_limits) and archived in Zenodo268. These repositories contain the (i) metadata (.csv), (ii) raw data (.csv), (iii) curated data (.csv), (iv) code detailing the data curation (.Rmd), (v) code for producing the figures in this data descriptor (.Rmd), (vi) supplementary data (.csv) and phylogenetic tree (.tre) used to build the figures in this data descriptor, and vii) a bibliographic file containing all the references cited in the database (.RIS). Data records are under a CC-BY license that enables reuse with attribution of this data descriptor, and we encourage the citation of original data sources.
Technical Validation
We systematically reviewed >4000 studies in five databases, and seven languages, using a transparent and reproducible workflow. However, we acknowledge that searches in Google Scholar may not be entirely reproducible. In fact, Google Scholar’s updating practices are not publicly available46, and results may vary over time. However, Google Scholar is still deemed as a complementary source of evidence to systematic reviews47,268, and was the best available tool, to our knowledge, for accessing materials in languages other than English.
We are confident to have obtained a representative and nearly comprehensive sample of the literature on amphibian upper thermal limits. Comparisons with previously published databases show that our synthesis (213 studies retrieved from searches in five databases and seven languages) has greatly expanded the current state of knowledge in the field. For instance, the Globtherm database24 contains data for 113 species (20 studies retrieved from English searches in one database), whereas the synthesis by Rohr and colleagues16 contains data for 251 species (85 studies retrieved from undescribed searches). However, it is important to note that our database still suffers from both taxonomic and geographic biases in sampling (Figs. 3–4). Caecilians (Gymnophiona), an entire order of amphibians, are not represented in our data (Fig. 3). Furthermore, we found very little published evidence from high latitudes, across the African continent, and in most of Asia (Fig. 4). While amphibian species richness is expected to be lower at high latitudes, we expected to retrieve more data from species native to Africa and Asia, where amphibian diversity is high43. We encourage researchers possessing relevant data to contact us and/or post an issue in Github to update the database.
Different authors extracted the data from original studies, which may have introduced some errors. Therefore, all data were cross-checked and standardized by the data extraction leader, PPottier. Data were explored in R to identify mistakes that occurred during data extraction. Data that were deemed as having procedural concerns (e.g., unclear acclimation conditions, exposure to UV radiations) were flagged, and removed during data curation. We also extracted measures of data dispersion around mean estimates (i.e., standard deviation, standard error) to help infer which estimates are the most representative of population estimates. While 25.1% of the estimates had no measures of dispersion, these could be estimated using data imputation procedures e.g.269,270. We also extracted information about factors that may generate data heterogeneity, or potential confounders. We encourage researchers to evaluate the comparability of the estimates they use in comparative analyses (see Usage Notes).
Usage Notes
We provide both the raw and curated versions of our database. For most questions in ecophysiology, we recommend using the curated database, as it does not contain animals exposed to hormones, chemicals, UV radiation, or pathogens; data having procedural concerns (e.g., unclear acclimation conditions, survival data converted to CTmax estimates); extinct species; and species not described at the species level. However, the raw data, or some subsets of it, could be used to address specific questions, such as whether chemical concentrations influence the capacity for amphibians to tolerate heat.
Our data also contains intrinsic sources of non-independence. We extracted multiple estimates per study, species, population, and cohort (e.g., multiple endpoints assessed on the same animals). Species also vary in terms of their evolutionary relatedness, and we recommend performing phylogenetically-informed syntheses. The compatibility of our database with the phylogeny from Jetz and Pyron265 should facilitate this task. We recommend using a hierarchical random effect structure to account for, quantify, and decompose the variance explained by those different components34.
Because upper thermal limits are plastic and vary with acclimation temperatures14,15,16,17,18, it is important to account for this variation in future studies. We recommend using this variable as a fixed factor and consider accounting for non-linear relationships between upper thermal limits and acclimation temperatures10. For a meta-analysis, one may convert the database to an ‘effect size’ format (sensu Schwanz et al.267) and use stepwise comparisons between acclimation temperatures as effect sizes14,271. Other variables affecting upper thermal limits include geographical origin9,272, ontogeny11,14,273, assay duration274,275, upper thermal limit metrics10, body size16,26,35, sex271,276, and photoperiod111. We recommend quantifying the variation arising from those variables and considering their inclusion in statistical models. Finally, we recommend weighing estimates by their precision to make the estimates more precise34,37, and more representative of the “true” populations sampled in statistical analysis (e.g., meta-analysis and comparative analysis). While not all our estimates contain a measure of dispersion, data imputation procedures can be used to retrieve missing standard deviations269,270.
Finally, we encourage users’ feedback to maintain the quality and usability of the database. We welcome the reporting of potential errors and encourage users possessing relevant data to update the database to increase its geographical and taxonomical coverage. User feedback can be issued at the GitHub repository (https://github.com/p-pottier/Amphibian_upper_thermal_limits).
Code availability
The code used to curate the data and produced the figures in this data descriptor are available at Zenodo268. All data manipulations were performed using the R statistical environment6 (v. 4.1.3) and the tidyverse package277 (v. 1.3.1). Figures were built upon the ggspatial278 (v. 1.1.5), maps279 (v. 3.3.0), ggtree280 (v. 3.1.1), ggtreeExtra281 (v. 1.3.3), ggnewscale282 (v. 0.4.5), patchwork283 (v. 1.1.1), and ape284 (v. 5.5) packages.
References
Arias, P. A. et al. Technical summary. in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (2021).
Pinsky, M. L., Eikeset, A. M., McCauley, D. J., Payne, J. L. & Sunday, J. M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2, 686–690 (2012).
Fry, F. Effects of the environment on animal activity. Publ. Ontario Fish. Res. Lab. 55, 1–62 (1947).
Lutterschmidt, W. I. & Hutchison, V. H. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574, https://doi.org/10.1139/z97-783 (2011).
Rezende, E. L., Castañeda, L. E. & Santos, M. Tolerance landscapes in thermal ecology. Funct. Ecol. 28, 799–809 (2014).
Bozinovic, F., Calosi, P. & Spicer, J. I. Physiological correlates of geographic range in animals. Annu. Rev. Ecol. Evol. S. 42, 155–179 (2011).
Sunday, J. M. et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 111, 5610–5615 (2014).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 105, 6668–6672 (2008).
Comte, L. & Olden, J. D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Chang. 7, 718–722 (2017).
Dahlke, F. T., Wohlrab, S., Butzin, M. & Pörtner, H.-O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369, 65–70 (2020).
Pottier, P., Burke, S., Drobniak, S. M. & Nakagawa, S. Methodological inconsistencies define thermal bottlenecks in fish life cycle: a comment on Dahlke et al. 2020. Evol. Ecol. 36, 287–292 (2022).
Dahlke, F., Butzin, M., Wohlrab, S. & Pörtner, H.-O. Reply to: methodological inconsistencies define thermal bottlenecks in fish life cycle. Evol. Ecol. 36, 293–298 (2022).
Pottier, P. et al. Developmental plasticity in thermal tolerance: Ontogenetic variation, persistence, and future directions. Ecol. Lett. (2022).
Morley, S. A., Peck, L. S., Sunday, J. M., Heiser, S. & Bates, A. E. Physiological acclimation and persistence of ectothermic species under extreme heat events. Glob. Ecol. Biogeogr. 28, 1018–1037 (2019).
Rohr, J. R. et al. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425–1439 (2018).
Gunderson, A. R. & Stillman, J. H. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B-Biol. Sci. 282, 20150401 (2015).
Weaving, H., Terblanche, J. S., Pottier, P. & English, S. Meta-analysis reveals weak but pervasive plasticity in insect thermal limits. Nat. Commun. 13, 1–11 (2022).
Araújo, M. B. et al. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219 (2013).
Bennett, J. M. et al. The evolution of critical thermal limits of life on Earth. Nat. Commun. 12, 1198 (2021).
Hoffmann, A. A., Chown, S. L. & Clusella-Trullas, S. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934–949 (2013).
Kellermann, V. et al. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl. Acad. Sci. USA 109, 16228–16233 (2012).
Morgan, R., Finnøen, M. H., Jensen, H., Pélabon, C. & Jutfelt, F. Low potential for evolutionary rescue from climate change in a tropical fish. Proc. Natl. Acad. Sci. USA 117, 33365–33372 (2020).
Bennett, J. M. et al. GlobTherm, a global database on thermal tolerances for aquatic and terrestrial organisms. Sci. Data 5, 180022 (2018).
Leiva, F. P., Calosi, P. & Verberk, W. C. E. P. Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water- and air-breathers. Philos. Trans. R. Soc. B-Biol. Sci. 374, 20190035 (2019).
Clusella-Trullas, S., Blackburn, T. M. & Chown, S. L. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751 (2011).
Nakagawa, S. & Freckleton, R. P. Missing inaction: the dangers of ignoring missing data. Trends Ecol. Evol. 23, 592–596 (2008).
Johnson, T. F., Isaac, N. J. B., Paviolo, A. & González-Suárez, M. Handling missing values in trait data. Glob. Ecol. Biogeogr. 30, 51–62 (2021).
Foo, Y. Z., O’Dea, R. E., Koricheva, J., Nakagawa, S. & Lagisz, M. A practical guide to question formation, systematic searching and study screening for literature reviews in ecology and evolution. Methods Ecol. Evol. 12, 1705–1720 (2021).
Reboredo Segovia, A. L., Romano, D. & Armsworth, P. R. Who studies where? Boosting tropical conservation research where it is most needed. Front. Ecol. Environ. 18, 159–166 (2020).
White, C. R. et al. Geographical bias in physiological data limits predictions of global change impacts. Funct. Ecol. 35, 1572–1578 (2021).
Amano, T., González-Varo, J. P. & Sutherland, W. J. Languages are still a major barrier to global Science. PLoS Biol. 14, e2000933 (2016).
Bennett, S., Duarte, C. M., Marbà, N. & Wernberg, T. Integrating within-species variation in thermal physiology into climate change ecology. Philos. Trans. R. Soc. B-Biol. Sci. 374, 20180550 (2019).
Noble, D. W. A. et al. Meta-analytic approaches and effect sizes to account for ‘nuisance heterogeneity’ in comparative physiology. J. Exp. Biol. 225, jeb243225 (2022).
Peralta-Maraver, I. & Rezende, E. L. Heat tolerance in ectotherms scales predictably with body size. Nat. Clim. Chang. 11, 58–63 (2021).
McKenzie, D. J. et al. Intraspecific variation in tolerance of warming in fishes. J. Fish Biol. 98, 1536–1555 (2021).
Morrissey, M. B. Meta-analysis of magnitudes, differences and variation in evolutionary parameters. J. Evol. Biol. 29, 1882–1904 (2016).
Duffy, G. A., Kuyucu, A. C., Hoskins, J. L., Hay, E. M. & Chown, S. L. Adequate sample sizes for improved accuracy of thermal trait estimates. Funct. Ecol. 35, 2647–2662 (2021).
IUCN. The IUCN Red List of Threatened Species. https://www.iucnredlist.org (2021).
Harfoot, M. B. J. et al. Using the IUCN Red List to map threats to terrestrial vertebrates at global scale. Nat. Ecol. Evol. 5, 1510–1519 (2021).
Sodhi, N. K. et al. Measuring the meltdown: Drivers of global amphibian extinction and decline. PLoS One 3 (2008).
Nowakowski, A. J. et al. Tropical amphibians in shifting thermal landscapes under land-use and climate change. Conserv. Physiol. 31, 96–105 (2017).
Jenkins, C. N., Pimm, S. L. & Joppa, L. N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 110, E2602–E2610 (2013).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, 210 (2016).
Vera-Baceta, M.-A., Thelwall, M. & Kousha, K. Web of Science and Scopus language coverage. Scientometrics 121, 1803–1813 (2019).
Giustini, D. & Boulos, M. N. K. Google Scholar is not enough to be used alone for systematic reviews. Online J. Public Health Inform. 5, 214 (2013).
Haddaway, N. R., Collins, A. M., Coughlin, D. & Kirk, S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS One 10, e0138237 (2015).
Gusenbauer, M. & Haddaway, N. R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 11, 181–217 (2020).
Harzing, A. Publish or perish. Res. Int. Manag. Softw. Release 27 (2007).
Cheng, C.-B. A study of warming tolerance and thermal acclimation capacity of tadpoles in Taiwan. (Tunghai University, 2017).
Wu, Q.-H. & Hsieh, C.-H. Thermal tolerance and population genetics of Hynobius fuca. (Chinese Culture University, 2016).
Jørgensen, L. B., Malte, H., Ørsted, M., Klahn, N. A. & Overgaard, J. A unifying model to estimate thermal tolerance limits in ectotherms across static, dynamic and fluctuating exposures to thermal stress. Sci. Rep. 11, 1–14 (2021).
Agudelo-Cantero, G. A. & Navas, C. A. Interactive effects of experimental heating rates, ontogeny and body mass on the upper thermal limits of anuran larvae. J. Therm. Biol. 82, 43–51 (2019).
Alveal Riquelme, N. Relaciones entre la fisiología térmica y las características bioclimáticas de Rhinella spinulosa (Anura: Bufonidae) en Chile a través del enlace mecanicista de nicho térmico. (Universidad de Concepción, 2015).
Alves, M. Tolerância térmica em espécies de anuros neotropicais do gênero Dendropsophus Fitzinger, 1843 e efeito da temperatura na resposta à predação. (Universidade Estadual de Santa Cruz, 2016).
Anderson, R. C. O. & Andrade, D. V. Trading heat and hops for water: Dehydration effects on locomotor performance, thermal limits, and thermoregulatory behavior of a terrestrial toad. Ecol. Evol. 7, 9066–9075 (2017).
Aponte Gutiérrez, A. Endurecimiento térmico en Pristimantis medemi (Anura: Craugastoridae), en coberturas boscosas del Municipio de Villavicencio (Meta). (Universidad Nacional de Colombia, 2020).
Arrigada García, K. Conductas térmica en dos poblaciones de Batrachyla taeniata provenientes de la localidad de Ucúquer en la región de O’Higgins y de la localidad de Hualpén en la región del Bío-Bío (Universidad de Concepción, 2019).
Azambuja, G., Martins, I. K., Franco, J. L. & Santos, T. Gdos Effects of mancozeb on heat shock protein 70 (HSP70) and its relationship with the thermal physiology of Physalaemus henselii (Peters, 1872) tadpoles (Anura: Leptodactylidae). J. Therm. Biol. 98, 102911 (2021).
Bacigalupe, L. D. et al. Natural selection on plasticity of thermal traits in a highly seasonal environment. Evol. Appl. 11, 2004–2013 (2018).
Barria, A. M. & Bacigalupe, L. D. Intraspecific geographic variation in thermal limits and acclimatory capacity in a wide distributed endemic frog. J. Therm. Biol. 69, 254–260 (2017).
Beltrán, I., Ramírez-Castañeda, V., Rodríguez-López, C., Lasso, E. & Amézquita, A. Dealing with hot rocky environments: critical thermal maxima and locomotor performance in Leptodactylus lithonaetes (anura: Leptodactylidae). Herpetol. J. 29, 155–161 (2019).
Berkhouse, C. & Fries, J. Critical thermal maxima of juvenile and adult San Marcos salamanders (Eurycea nana). Southwest. Nat. 40, 430–434 (1995).
Blem, C. R., Ragan, C. A. & Scott, L. S. The thermal physiology of two sympatric treefrogs Hyla cinerea and Hyla chrysoscelis (Anura; Hylidae). Comp. Biochem. Physiol. 85, 563–570 (1986).
Bonino, M. F., Cruz, F. B. & Perotti, M. G. Does temperature at local scale explain thermal biology patterns of temperate tadpoles? J. Therm. Biol. 94 (2020).
Bovo, R. P. Fisiologia térmica e balanço hídrico em anfíbios anuros. (Universidad Estadual Paulista, 2015).
Brattstrom, B. H. Thermal acclimation in Australian amphibians. Comp. Biochem. Physiol. 35, 69–103 (1970).
Brattstrom, B. H. & Regal, P. Rate of thermal acclimation in the Mexican salamander. Chiropterotriton. Copeia 1965, 514–515 (1965).
Brattstrom, B. H. A preliminary review of the thermal requirements of amphibians. Ecology 44, 238–255 (1963).
Brattstrom, B. H. Thermal acclimation in anuran amphibians as a function of latitude and altitude. Comp. Biochem. Physiol. 24, 93–111 (1968).
Brattstrom, B. H. & Lawrence, P. The rate of thermal acclimation in anuran amphibians. Physiol. Zool. 35, 148–156 (1962).
Brown, H. A. The heat resistance of some anuran tadpoles (Hylidae and Pelobatidae). Copeia 1969, 138 (1969).
Burke, E. M. & Pough, F. H. The role of fatigue in temperature resistance of salamanders. J. Therm. Biol. 1, 163–167 (1976).
Burrowes, P. A., Navas, C. A., Jiménez-Robles, O., Delgado, P. & De La Riva, I. Climatic heterogeneity in the Bolivian andes: Are frogs trapped? S. Am. J. Herpetol. 18, 1–12 (2020).
Bury, R. B. Low thermal tolerances of stream amphibians in the Pacific Northwest: Implications for riparian and forest management. Appl. Herpetol. 5, 63–74 (2008).
Castellanos García, L. A. Days of futures past: Integrating physiology, microenvironments, and biogeographic history to predict response of frogs in neotropical dry-forest to global warming. (Universidad de los Andes, 2017).
Castro, B. Influence of environment on thermal ecology of direct-developing frogs (Anura: Craugastoridae: Pristimantis) in the eastern Andes of Colombia. (Universidad de los Andes, 2019).
Catenazzi, A., Lehr, E. & Vredenburg, V. T. Thermal physiology, disease, and amphibian declines on the eastern slopes of the Andes. Conserv. Biol. 28, 509–517 (2014).
Chang, L.-W. Heat tolerance and its plasticity in larval Bufo bankorensis from different altitudes. (National Cheng Kung University, 2002).
Chavez Landi, P. A. Fisiología térmica de un depredador Dasythemis sp. (Odonata: Libellulidae) y su presa Hypsiboas pellucens (Anura: Hylidae) y sus posibles implicaciones frente al cambio climático. (Pontificia Universidad Católica Del Ecuador, 2017).
Chen, T.-C., Kam, Y.-C. & Lin, Y.-S. Thermal physiology and reproductive phenology of Buergeria japonica (Rhacophoridae) breeding in a stream and a geothermal hotspring in Taiwan. Zool. Sci. 18, 591–596 (2001).
Cheng, Y.-J. Effect of salinity on the critical thermal maximum of tadpoles living in brackish water. (Tunghai University, 2017).
Christian, K. A., Nunez, F., Clos, L. & Diaz, L. Thermal relations of some tropical frogs along an altitudinal gradient. Biotropica 20, 236–239 (1988).
Claussen, D. L. The thermal relations of the tailed frog, Ascaphus truei, and the pacific treefrog, Hyla regilla. Comp. Biochem. Physiol. 44, 137–153 (1973).
Claussen, D. L. Thermal acclimation in ambystomatid salamanders. Comp. Biochem. Physiol. 58, 333–340 (1977).
Contreras Cisneros, J. Temperatura crítica máxima, tolerancia al frío y termopreferendum del tritón del Montseny (Calotriton arnoldii). (Universitat de Barcelona, 2019).
Contreras Oñate, S. Posible efecto de las temperaturas de aclimatación sobre las respuestas térmicas en temperaturas críticas máximas (TCmás) y mínimas (TCmín) de una población de Batrachyla taeniata (Universidad de Concepción, 2016).
Cooper, R. D. & Shaffer, H. B. Allele-specific expression and gene regulation help explain transgressive thermal tolerance in non-native hybrids of the endangered California tiger salamander (Ambystoma californiense). Mol. Ecol. 30, 987–1004 (2021).
Crow, J. C., Forstner, M. R. J., Ostr, K. G. & Tomasso, J. R. The role of temperature on survival and growth of the barton springs salamander (Eurycea sosorum). Herpetol. Conserv. Biol. 11, 328–334 (2016).
Cupp, P. V. Thermal tolerance of five salientian amphibians during development and metamorphosis. Herpetologica 36, 234–244 (1980).
Dabruzzi, T. F., Wygoda, M. L. & Bennett, W. A. Some like it hot: Heat tolerance of the crab-eating frog, Fejervarya cancrivora. Micronesica 43, 101–106 (2012).
Dainton, B. H. Heat tolerance and thyroid activity in developing tadpoles and juvenile adults of Xenopus laevis (Daudin). J. Therm. Biol. 16, 273–276 (1991).
Daniel, N. J. J. Impact of climate change on Singapore amphibians. (National University of Singapore, 2013).
Davies, S. J., McGeoch, M. A. & Clusella-Trullas, S. Plasticity of thermal tolerance and metabolism but not water loss in an invasive reed frog. Comp. Biochem. Physiol. 189, 11–20 (2015).
de Oliviera Anderson, R. C., Bovo, R. P. & Andrade, D. V. Seasonal variation in the thermal biology of a terrestrial toad, Rhinella icterica (Bufonidae), from the Brazilian Atlantic Forest. J. Therm. Biol. 74, 77–83 (2018).
de Vlaming, V. L. & Bury, R. B. Thermal selection in tadpoles of the tailed-frog. Ascaphus truei. J. Herpetol. 4, 179–189 (1970).
Delson, J. & Whitford, W. G. Critical thermal maxima in several life history stages in desert and montane populations of Ambystoma tigrinum. Herpetologica 29, 352–355 (1973).
Duarte, H. et al. Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Glob. Chang. Biol. 18, 412–421 (2012).
Duarte, H. S. A comparative study of the thermal tolerance of tadpoles of Iberian anurans. (Universidade de Lisboa, 2011).
Dunlap, D. Evidence for a daily rhythm of heat resistance in cricket frogs, Acris crepitans. Copeia. 4, 852–854 (1969).
Dunlap, D. G. Critical thermal maximum as a function of temperature of acclimation in two species of hylid frogs. Physiol. Zool. 41, 432–439 (1968).
Elwood, J. R. L. Variation in hsp70 levels and thermotolerance among terrestrial salamanders of the Plethodon glutinosus complex. (Drexel University, 2003).
Enriquez-Urzelai, U. et al. Ontogenetic reduction in thermal tolerance is not alleviated by earlier developmental acclimation in Rana temporaria. Oecologia 189, 385–394 (2019).
Enriquez-Urzelai, U. et al. The roles of acclimation and behaviour in buffering climate change impacts along elevational gradients. J. Anim. Ecol. 89, 1722–1734 (2020).
Erskine, D. J. & Hutchison, V. H. Reduced thermal tolerance in an amphibian treated with melatonin. J. Therm. Biol. 7, 121–123 (1982).
Escobar Serrano, D. Acclimation scope of the critical thermal limits in Agalychnis spurrelli (Hylidae) and Gastrotheca pseustes (Hemiphractidae) and their implications under climate change scenarios. (Pontificia Universidad Católica Del Ecuador, 2016).
Fan, X., Lei, H. & Lin, Z. Ontogenetic shifts in selected body temperature and thermal tolerance of the tiger frog. Hoplobatrachus chinensis. Acta Ecol. Sin. 32, 5574–5580 (2012).
Fan, X. L., Lin, Z. H. & Scheffers, B. R. Physiological, developmental, and behavioral plasticity in response to thermal acclimation. J. Therm. Biol. 97 (2021).
Fernández-Loras, A. et al. Infection with Batrachochytrium dendrobatidis lowers heat tolerance of tadpole hosts and cannot be cleared by brief exposure to CTmax. PLoS ONE 14 (2019).
Floyd, R. B. Ontogenetic change in the temperature tolerance of larval Bufo marinus (Anura: bufonidae). Comp. Biochem. Physiol. 75, 267–271 (1983).
Floyd, R. B. Effects of photoperiod and starvation on the temperature tolerance of larvae of the giant toad, Bufo marinus. Copeia 1985, 625–631 (1985).
Fong, S.-T. Thermal tolerance of adult Asiatic painted frog Kaloula pulchra from different populations. (National University of Tainan, 2014).
Frishkoff, L. O., Hadly, E. A. & Daily, G. C. Thermal niche predicts tolerance to habitat conversion in tropical amphibians and reptiles. Glob. Chang. Biol. 21, 3901–3916 (2015).
Frost, J. S. & Martin, E. W. A comparison of distribution and high temperature tolerance in Bufo americanus and Bufo woodhousii fowleri. Copeia 1971, 750 (1971).
Gatz, A. J. Critical thermal maxima of Ambystoma maculatum (Shaw) and Ambystoma jeffersonianum (Green) in relation to time of breeding. Herpetologica 27, 157–160 (1971).
Gatz, A. J. Intraspecific variations in critical thermal maxima of Ambystoma maculatum. Herpetologica 29, 264–268 (1973).
Geise, W. & Linsenmair, K. E. Adaptations of the reed frog Hyperolius viridiflavus (Amphibia, Anura, Hyperoliidae) to its arid environment - IV. Ecological significance of water economy with comments on thermoregulation and energy allocation. Oecologia 77, 327–338 (1988).
González-del-Pliego, P. et al. Thermal tolerance and the importance of microhabitats for Andean frogs in the context of land use and climate change. J. Anim. Ecol. 89, 2451–2460 (2020).
Gouveia, S. F. et al. Climatic niche at physiological and macroecological scales: The thermal tolerance-geographical range interface and niche dimensionality. Glob. Ecol. Biogeogr. 23, 446–456 (2014).
Gray, R. Lack of physiological differentiation in three color morphs of the cricket frog (Acris crepitans) in Illinois. Trans. Ill. State Acad. Sci. 70, 73–79 (1977).
Greenspan, S. E. et al. Infection increases vulnerability to climate change via effects on host thermal tolerance. Sci. Rep. 7 (2017).
Guevara-Molina, E. C., Gomes, F. R. & Camacho, A. Effects of dehydration on thermoregulatory behavior and thermal tolerance limits of Rana catesbeiana (Shaw, 1802). J. Therm. Biol. 93 (2020).
Gutiérrez Pesquera, L. Una valoración macrofisiológica de la vulnerabilidad al calentamiento global. Análisis de los límites de tolerancia térmica en comunidades de anfibios en gradients latitudinales y altitudinales. (Pontificia Universidad Católica Del Ecuador, 2015).
Gutiérrez Pesquera, M. Thermal tolerance across latitudinal and altitudinal gradients in tadpoles. (Universidad de Sevilla, 2016).
Gutiérrez-Pesquera, L. M. et al. Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. J. Biogeogr. 43, 1166–1178 (2016).
Gvoždík, L., Puky, M. & Šugerková, M. Acclimation is beneficial at extreme test temperatures in the Danube crested newt, Triturus dobrogicus (Caudata, Salamandridae). Bio. J. Linn. Soc. 90, 627–636 (2007).
Heatwole, H., De Austin, S. B. & Herrero, R. Heat tolerances of tadpoles of two species of tropical anurans. Comp. Biochem. Physiol. 27, 807–815 (1968).
Heatwole, H., Mercado, N. & Ortiz, E. Comparison of critical thermal maxima of two species of Puerto Rican frogs of the genus. Eleutherodactylus. Physiol. Zool. 38, 1–8 (1965).
Holzman, N. & McManus, J. J. Effects of acclimation on metabolic rate and thermal tolerance in the carpenter frog. Rana vergatipes. Comp. Biochem. Physiol. 45, 833–842 (1973).
Hoppe, D. M. Thermal tolerance in tadpoles of the chorus frog Pseudacris triseriata. Herpetologica 34, 318–321 (1978).
Hou, P.-C. Thermal tolerance and preference in the adult amphibians from different altitudinal LTER sites. (National Cheng Kung University, 2003).
Howard, J. H., Wallace, R. L. & Stauffer, J. R. Jr Critical thermal maxima in populations of Ambystoma macrodactylum from different elevations. J. Herpetol. 17, 400–402 (1983).
Hutchison, V. H. & Ritchart, J. P. Annual cycle of thermal tolerance in the salamander. Necturus maculosus. J. Herpetol. 23, 73–76 (1989).
Hutchison, V. H. The distribution and ecology of the cave salamander, Eurycea lucifuga. Ecol. Monogr. 28, 2–20 (1958).
Hutchison, V. H. Critical thermal maxima in salamanders. Physiol. Zool. 34, 92–125 (1961).
Hutchison, V. H., Engbretson, G. & Turney, D. Thermal acclimation and tolerance in the hellbender, Cryptobranchus alleganiensis. Copeia 1973, 805–807 (1973).
Hutchison, V. H. & Rowlan, S. D. Thermal acclimation and tolerance in the mudpuppy. Necturus maculosus. J. Herpetol. 9, 367–368 (1975).
Jiang, S., Yu, P. & Hu, Q. A study on the critical thermal maxima of five species of salamanders of China. Acta Herpetol. Sin. 6, 56–62 (1987).
John-Alder, H. B., Morin, P. J. & Lawler, S. Thermal physiology, phenology, and distribution of tree frogs. Am. Nat. 132, 506–520 (1988).
Johnson, C. R. Daily variation in the thermal tolerance of Litoria caerulea (Anura: Hylidae). Comp. Biochem. Physiol. 40, 1109–1111 (1971).
Johnson, C. R. Thermal relations and water balance in the day frog, Taudactylus diurnus, from an Australian rain forest. Aust. J. Zool. 19, 35–39 (1971).
Johnson, C. R. Diel variation in the thermal tolerance of Litoria gracilenta (Anura: Hylidae). Comp. Biochem. Physiol. 41, 727–730 (1972).
Johnson, C. R. & Prine, J. E. The effects of sublethal concentrations of organophosphorus insecticides and an insect growth regulator on temperature tolerance in hydrated and dehydrated juvenile western toads. Bufo boreas. Comp. Biochem. Physiol. 53, 147–149 (1976).
Johnson, C. R. Observations on body temperatures, critical thermal maxima and tolerance to water loss in the Australian hylid, Hyla caerulea (White). Proc. R. Soc. Qld. 82, 47–50 (1970).
Johnson, C. R. Thermal relations and daily variation in the thermal tolerance in. Bufo marinus. J. Herpetol. 6, 35 (1972).
Johnson, C. Thermal relations in some southern and eastern Australian anurans. Proc. R. Soc. Qld. 82, 87–94 (1971).
Johnson, C. The effects of five organophosphorus insecticides on thermal stress in tadpoles of the Pacific tree frog. Hyla regilla. Zool. J. Linn. Soc. 69, 143–147 (1980).
Katzenberger, M., Duarte, H., Relyea, R., Beltrán, J. F. & Tejedo, M. Variation in upper thermal tolerance among 19 species from temperate wetlands. J. Therm. Biol. 96 (2021).
Katzenberger, M. et al. Swimming with predators and pesticides: How environmental stressors affect the thermal physiology of tadpoles. PLoS ONE 9 (2014).
Katzenberger, M., Hammond, J., Tejedo, M. & Relyea, R. Source of environmental data and warming tolerance estimation in six species of North American larval anurans. J. Therm. Biol. 76, 171–178 (2018).
Katzenberger, M. Thermal tolerance and sensitivity of amphibian larvae from Palearctic and Neotropical communities. (Universidade de Lisboa, 2013).
Katzenberger, M. Impact of global warming in holarctic and neotropical communities of amphibians. (Universidad de Sevilla, 2014).
Kern, P., Cramp, R. L. & Franklin, C. E. Temperature and UV-B-insensitive performance in tadpoles of the ornate burrowing frog: An ephemeral pond specialist. J. Exp. Biol. 217, 1246–1252 (2014).
Kern, P., Cramp, R. L., Seebacher, F., Ghanizadeh Kazerouni, E. & Franklin, C. E. Plasticity of protective mechanisms only partially explains interactive effects of temperature and UVR on upper thermal limits. Comp. Biochem. Physiol. 190, 75–82 (2015).
Kern, P., Cramp, R. L. & Franklin, C. E. Physiological responses of ectotherms to daily temperature variation. J. Exp. Biol. 218, 3068–3076 (2015).
Komaki, S., Igawa, T., Lin, S.-M. & Sumida, M. Salinity and thermal tolerance of Japanese stream tree frog (Buergeria japonica) tadpoles from island populations. Herpetol. J. 26, 207–211 (2016).
Komaki, S., Lau, Q. & Igawa, T. Living in a Japanese onsen: Field observations and physiological measurements of hot spring amphibian tadpoles. Buergeria japonica. Amphib. Reptil. 37, 311–314 (2016).
Krakauer, T. Tolerance limits of the toad, Bufo marinus, in South Florida. Comp. Biochem. Physiol. 33, 15–26 (1970).
Kurabayashi, A. et al. Improved transport of the model amphibian, Xenopus tropicalis, and its viable temperature for transport. Curr. Herpetol. 33, 75–87 (2014).
Lau, E. T. C., Leung, K. M. Y. & Karraker, N. E. Native amphibian larvae exhibit higher upper thermal limits but lower performance than their introduced predator. Gambusia affinis. J. Therm. Biol. 81, 154–161 (2019).
Layne, J. R. & Claussen, D. L. Seasonal variation in the thermal acclimation of critical thermal maxima (CTMax) and minima (CTMin) in the salamander. Eurycea bislineata. J. Therm. Biol. 7, 29–33 (1982).
Layne, J. R. & Claussen, D. L. The time courses of CTMax and CTMin acclimation in the salamander. Desmognathus fuscus. J. Therm. Biol. 7, 139–141 (1982).
Lee, P.-T. Acidic effect on tadpoles living in container habitats. (Tunghai University, 2019).
Longhini, L. S., De Almeida Prado, C. P., Bícego, K. C., Zena, L. A. & Gargaglioni, L. H. Measuring cardiorespiratory variables on small tadpoles using a non-invasive methodology. Rev. Cuba. Investig. Biomed. 38 (2019).
López Rosero, A. C. Ontogenetic variation of thermal tolerance in two anuran species of Ecuador: Gastrotheca pseustes (Hemiphractidae) and Smilisca phaeota (Hylidae) and their relative vulnerability to environmental temperature change. (Pontificia Universidad Católica Del Ecuador, 2015).
Lotshaw, D. P. Temperature adaptation and effects of thermal acclimation in Rana sylvatica and Rana catesbeiana. Comp. Biochem. Physiol. 56, 287–294 (1977).
Lu, H.-L., Wu, Q., Geng, J. & Dang, W. Swimming performance and thermal resistance of juvenile and adult newts acclimated to different temperatures. Acta Herpetol. 11, 189–195 (2016).
Lu, H. L., Geng, J., Xu, W., Ping, J. & Zhang, Y. P. Physiological response and changes in swimming performance after thermal acclimation in juvenile chinese fire-belly newts, Cynops orientalis. Acta Ecol. Sin. 37, 1603–1610 (2017).
Lutterschmidt, W. I. & Hutchison, V. H. The critical thermal maximum: Data to support the onset of spasms as the definitive end point. Can. J. Zool. 75, 1553–1560 (1997).
Madalozzo, B. Variação latitudinal nos limites de tolerância e plasticidade térmica em anfíbios em um cenário de mudanças climáticas: efeito dos micro-habitats, sazonalidade e filogenia. (Universidade Federal de Santa Maria, 2018).
Mahoney, J. J. & Hutchison, V. H. Photoperiod acclimation and 24-hour variations in the critical thermal maxima of a tropical and a temperate frog. Oecologia 2, 143–161 (1969).
Maness, J. D. & Hutchison, V. H. Acute adjustment of thermal tolerance in vertebrate ectotherms following exposure to critical thermal maxima. J. Therm. Biol. 5, 225–233 (1980).
Manis, M. L. & Claussen, D. L. Environmental and genetic influences on the thermal physiology of Rana sylvatica. J. Therm. Biol. 11, 31–36 (1986).
Markle, T. M. & Kozak, K. H. Low acclimation capacity of narrow-ranging thermal specialists exposes susceptibility to global climate change. Ecol. Evol. 8, 4644–4656 (2018).
Marshall, E. & Grigg, G. C. Acclimation of CTM, LD50, and rapid loss of acclimation of thermal preferendum in tadpoles of Limnodynastes peronii (Anura, Myobatrachidae). Aust. Zool. 20, 447–456 (1980).
Mathias, J. H. The Comparative ecologies of two species of Amphibia (B. bufo and B. calamita) on the Ainsdale Sand Dunes National Nature Reserve. (The University of Manchester, 1971).
McManus, J. J. & Nellis, D. W. The critical thermal maximum of the marine toad, Bufo marinus. Caribb. J. Sci. 15, 67–70 (1975).
Menke, M. E. & Claussen, D. L. Thermal acclimation and hardening in tadpoles of the bullfrog, Rana catesbeiana. J. Therm. Biol. 7, 215–219 (1982).
Merino-Viteri, A. R. The vulnerability of microhylid frogs, Cophixalus spp., to climate change in the Australian Wet Tropics. (James Cook University, 2018).
Messerman, A. F. Tales of an ‘invisible’ life stage: Survival and physiology among terrestrial juvenile ambystomatid salamanders. (University of Missouri, 2019).
Meza-Parral, Y., García-Robledo, C., Pineda, E., Escobar, F. & Donnelly, M. A. Standardized ethograms and a device for assessing amphibian thermal responses in a warming world. J. Therm. Biol. 89 (2020).
Miller, K. & Packard, G. C. Critical thermal maximum: Ecotypic variation between montane and piedmont chorus frogs (Pseudacris triseriata, Hylidae). Experientia 30, 355–356 (1974).
Miller, K. & Packard, G. C. An altitudinal cline in critical thermal maxima of chorus frogs (Pseudacris triseriata). Am. Nat. 111, 267–277 (1977).
Mueller, C. A., Bucsky, J., Korito, L. & Manzanares, S. Immediate and persistent effects of temperature on oxygen consumption and thermal tolerance in embryos and larvae of the baja California chorus frog, Pseudacris hypochondriaca. Front. Physiol. 10 (2019).
Navas, C. A., Antoniazzi, M. M., Carvalho, J. E., Suzuki, H. & Jared, C. Physiological basis for diurnal activity in dispersing juvenile Bufo granulosus in the Caatinga, a Brazilian semi-arid environment. Comp. Biochem. Physiol. 147, 647–657 (2007).
Navas, C. A., Úbeda, C. A., Logares, R. & Jara, F. G. Thermal tolerances in tadpoles of three species of Patagonian anurans. S. Am. J. Herpetol. 5, 89–96 (2010).
Nietfeldt, J. W., Jones, S. M., Droge, D. L. & Ballinger, R. E. Rate of thermal acclimation of larval Ambystoma tigrinum. J. Herpetol. 14, 209–211 (1980).
Nol, R. & Ultsch, G. R. The roles of temperature and dissolved oxygen in microhabitat selection by the tadpoles of a frog (Rana pipiens) and a toad (Bufo terrestris). Copeia 1981, 645–652 (1981).
Novarro, A. J. Thermal physiology in a widespread lungless salamander. (University of Maryland, 2018).
Nowakowski, A. J. et al. Thermal biology mediates responses of amphibians and reptiles to habitat modification. Ecol. Lett. 21, 345–355 (2018).
Orille, A. C., McWhinnie, R. B., Brady, S. P. & Raffel, T. R. Positive effects of acclimation temperature on the critical thermal maxima of Ambystoma mexicanum and Xenopus laevis. J. Herpetol. 54, 289–292 (2020).
Oyamaguchi, H. M. et al. Thermal sensitivity of a neotropical amphibian (Engystomops pustulosus) and its vulnerability to climate change. Biotropica 50, 326–337 (2018).
Paez Vacas, M. I. Mechanisms of population divergence along elevational gradients in the tropics. (Colorado State University, 2016).
Paulson, B. K. & Hutchison, V. H. Blood changes in Bufo cognatus following acute heat stress. Comp. Biochem. Physiol. 87, 461–466 (1987).
Paulson, B. & Hutchison, V. Origin of the stimulus for muscular spasms at the critical thermal maximum in anurans. Copeia 810–813 (1987).
Percino-Daniel, R. et al. Environmental heterogeneity shapes physiological traits in tropical direct-developing frogs. Ecol. Evol. (2021).
Perotti, M. G., Bonino, M. F., Ferraro, D. & Cruz, F. B. How sensitive are temperate tadpoles to climate change? The use of thermal physiology and niche model tools to assess vulnerability. Zoology 127, 95–105 (2018).
Pintanel, P., Tejedo, M., Almeida-Reinoso, F., Merino-Viteri, A. & Gutiérrez-Pesquera, L. M. Critical thermal limits do not vary between wild-caught and captive-bred tadpoles of Agalychnis spurrelli (Anura: Hylidae). Diversity 12, 43 (2020).
Pintanel, P., Tejedo, M., Ron, S. R., Llorente, G. A. & Merino-Viteri, A. Elevational and microclimatic drivers of thermal tolerance in Andean Pristimantis frogs. J. Biogeogr. 46, 1664–1675 (2019).
Pintanel, P. Thermal adaptation of amphibians in tropical mountains. Consequences of global warming. (Universitat de Barcelona, 2018).
Pintanel, P., Tejedo, M., Salinas-Ivanenko, S., Jervis, P. & Merino-Viteri, A. Predators like it hot: Thermal mismatch in a predator-prey system across an elevational tropical gradient. J. Anim. Ecol. 90, 1985–1995 (2021).
Pough, F. H. Natural daily temperature acclimation of eastern red efts, Notophthalmus v. viridescens (Rafinesque) (Amphibia: Caudata). Comp. Biochem. Physiol. 47, 71–78 (1974).
Pough, F. H., Stewart, M. M. & Thomas, R. G. Physiological basis of habitat partitioning in Jamaican. Eleutherodactylus. Oecologia 27, 285–293 (1977).
Quiroga, L. B., Sanabria, E. A., Fornés, M. W., Bustos, D. A. & Tejedo, M. Sublethal concentrations of chlorpyrifos induce changes in the thermal sensitivity and tolerance of anuran tadpoles in the toad Rhinella arenarum? Chemosphere 219, 671–677 (2019).
Rausch, C. The thermal ecology of the red-spotted toad, Bufo punctatus, across life history. (University of Nevada, 2007).
Reichenbach, N. & Brophy, T. R. Natural history of the peaks of otter salamander (Plethodon hubrichti) along an elevational gradient. Herpetol. Bull. 141, 7–15 (2017).
Reider, K. E., Larson, D. J., Barnes, B. M. & Donnelly, M. A. Thermal adaptations to extreme freeze–thaw cycles in the high tropical Andes. Biotropica 53, 296–306 (2021).
Richter-Boix, A. et al. Local divergence of thermal reaction norms among amphibian populations is affected by pond temperature variation. Evolution 69, 2210–2226 (2015).
Riquelme, N. A., Díaz-Páez, H. & Ortiz, J. C. Thermal tolerance in the Andean toad Rhinella spinulosa (Anura: Bufonidae) at three sites located along a latitudinal gradient in Chile. J. Therm. Biol. 60, 237–245 (2016).
Ritchart, J. P. & Hutchison, V. H. The effects of ATP and cAMP on the thermal tolerance of the mudpuppy. Necturus maculosus. J. Therm. Biol. 11, 47–51 (1986).
Rivera-Burgos, A. C. Habitat suitability for Eleutherodactylus frogs in Puerto Rico: Indexing occupancy, abundance and reproduction to climatic and habitat characteristics. (North Carolina State University, 2019).
Rivera-Ordonez, J. M., Nowakowski, A. J., Manansala, A., Thompson, M. E. & Todd, B. D. Thermal niche variation among individuals of the poison frog, Oophaga pumilio, in forest and converted habitats. Biotropica 51, 747–756 (2019).
Romero Barreto, P. Requerimientos fisiológicos y microambientales de dos especies de anfibios (Scinax ruber e Hyloxalus yasuni) del bosque tropical de Yasuní y sus implicaciones ante el cambio climático. (Pontificia Universidad Católica Del Ecuador, 2013).
Ruiz-Aravena, M. et al. Impact of global warming at the range margins: Phenotypic plasticity and behavioral thermoregulation will buffer an endemic amphibian. Ecol. Evol. 4, 4467–4475 (2014).
Ruthsatz, K. et al. Thyroid hormone levels and temperature during development alter thermal tolerance and energetics of Xenopus laevis larvae. Conserv. Physiol. 6 (2018).
Ruthsatz, K. et al. Post-metamorphic carry-over effects of altered thyroid hormone level and developmental temperature: physiological plasticity and body condition at two life stages in Rana temporaria. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 190, 297–315 (2020).
Rutledge, P. S., Spotila, J. R. & Easton, D. P. Heat hardening in response to two types of heat shock in the lungless salamanders Eurycea bislineata and Desmognathus ochrophaeus. J. Therm. Biol. 12, 235–241 (1987).
Sanabria, E. et al. Effect of salinity on locomotor performance and thermal extremes of metamorphic Andean Toads (Rhinella spinulosa) from Monte Desert, Argentina. J. Therm. Biol. 74, 195–200 (2018).
Sanabria, E. A., González, E., Quiroga, L. B. & Tejedo, M. Vulnerability to warming in a desert amphibian tadpole community: the role of interpopulational variation. J. Zool. 313, 283–296 (2021).
Sanabria, E. A. & Quiroga, L. B. Change in the thermal biology of tadpoles of Odontophrynus occidentalis from the Monte desert, Argentina: Responses to photoperiod. J. Therm. Biol. 36, 288–291 (2011).
Sanabria, E. A., Quiroga, L. B., González, E., Moreno, D. & Cataldo, A. Thermal parameters and locomotor performance in juvenile of Pleurodema nebulosum (Anura: Leptodactylidae) from the Monte Desert. J. Therm. Biol. 38, 390–395 (2013).
Sanabria, E. A., Quiroga, L. B. & Martino, A. L. Seasonal changes in the thermal tolerances of the toad Rhinella arenarum (Bufonidae) in the Monte Desert of Argentina. J. Therm. Biol. 37, 409–412 (2012).
Sanabria, E. A., Quiroga, L. B. & Martino, A. L. Seasonal Changes in the thermal tolerances of Odontophrynus occidentalis (Berg, 1896) (Anura: Cycloramphidae). Belg. J. Zool. 143, 23–29 (2013).
Sanabria, E. A. et al. Thermal ecology of the post-metamorphic Andean toad (Rhinella spinulosa) at elevation in the monte desert, Argentina. J. Therm. Biol. 52, 52–57 (2015).
Sanabria, E. A., Vaira, M., Quiroga, L. B., Akmentins, M. S. & Pereyra, L. C. Variation of thermal parameters in two different color morphs of a diurnal poison toad, Melanophryniscus rubriventris (Anura: Bufonidae). J. Therm. Biol. 41, 1–5 (2014).
Sanabria, E. A. & Quiroga, L. B. Thermal parameters changes in males of Rhinella arenarum (Anura: Bufonidae) related to reproductive periods. Rev. Biol. Trop. 59, 347–353 (2011).
Scheffers, B. R. et al. Thermal buffering of microhabitats is a critical factor mediating warming vulnerability of frogs in the Philippine biodiversity hotspot. Biotropica 45, 628–635 (2013).
Scheffers, B. R., Edwards, D. P., Diesmos, A., Williams, S. E. & Evans, T. A. Microhabitats reduce animal’s exposure to climate extremes. Glob. Chang. Biol. 20, 495–503 (2014).
Schmid, W. D. High temperature tolerances of Bufo Hemiophrys and Bufo Cognatus. Ecology 46, 559–560 (1965).
Sealander, J. A. & West, B. W. Critical thermal maxima of some Arkansas salamanders in relation to thermal acclimation. Herpetologica 25, 122–124 (1969).
Seibel, R. V. Variables affecting the critical thermal maximum of the leopard frog, Rana pipiens Schreber. Herpetologica 26, 208–213 (1970).
Sherman, E. Ontogenetic change in thermal tolerance of the toad Bufo woodhousii fowleri. Comp. Biochem. Physiol. 65, 227–230 (1980).
Sherman, E. Thermal biology of newts (Notophthalmus viridescens) chronically infected with a naturally occurring pathogen. J. Therm. Biol. 33, 27–31 (2008).
Sherman, E., Baldwin, L., Fernandez, G. & Deurell, E. Fever and thermal tolerance in the toad Bufo marinus. J. Therm. Biol. 16, 297–301 (1991).
Sherman, E. & Levitis, D. Heat hardening as a function of developmental stage in larval and juvenile Bufo americanus and Xenopus laevis. J. Therm. Biol. 28, 373–380 (2003).
Shi, L., Zhao, L., Ma, X. & Ma, X. Selected body temperature and thermal tolerance of tadpoles of two frog species (Fejervarya limnocharis and Microhyla ornata) acclimated under different thermal conditions. Acta Ecol. Sin. 32, 0465–0471 (2012).
Simon, M. N., Ribeiro, P. L. & Navas, C. A. Upper thermal tolerance plasticity in tropical amphibian species from contrasting habitats: Implications for warming impact prediction. J. Therm. Biol. 48, 36–44 (2015).
Simon, M. Plasticidade fenotípica em relação à temperatura de larvas de Rhinella (Anura: Bufonidae) da caatinga e da floresta Atlântica. (Universidade de Sao Paulo, 2010).
Skelly, D. K. & Freidenburg, L. K. Effects of beaver on the thermal biology of an amphibian. Ecol. Lett. 3, 483–486 (2000).
Sos, T. Thermoconformity even in hot small temporary water bodies: a case study in yellow-bellied toad (Bombina v. variegata). Herpetol. Rom. 1, 1–11 (2007).
Spotila, J. R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 42, 95–125 (1972).
Tracy, C. R., Christian, K. A., Betts, G. & Tracy, C. R. Body temperature and resistance to evaporative water loss in tropical Australian frogs. Comp. Biochem. Physiol. 150, 102–108 (2008).
Turriago, J. L., Parra, C. A. & Bernal, M. H. Upper thermal tolerance in anuran embryos and tadpoles at constant and variable peak temperatures. Can. J. Zool. 93, 267–272 (2015).
Vidal, M. A., Novoa-Muñoz, F., Werner, E., Torres, C. & Nova, R. Modeling warming predicts a physiological threshold for the extinction of the living fossil frog Calyptocephalella gayi. J. Therm. Biol. 69, 110–117 (2017).
von May, R. et al. Divergence of thermal physiological traits in terrestrial breeding frogs along a tropical elevational gradient. Ecol. Evol. 7, 3257–3267 (2017).
von May, R. et al. Thermal physiological traits in tropical lowland amphibians: Vulnerability to climate warming and cooling. PLoS ONE 14 (2019).
Wagener, C., Kruger, N. & Measey, J. Progeny of Xenopus laevis from altitudinal extremes display adaptive physiological performance. J. Exp. Biol. 224 (2021).
Wang, H. & Wang, L. Thermal adaptation of the common giant toad (Bufo gargarizans) at different earlier developmental stages. J. Agric. Univ. Hebei 31, 79–83 (2008).
Wang, L. The effects of constant and variable thermal acclimation on thermal tolerance of the common giant toad tadpoles (Bufo gargarizans). Acta Ecol. Sin. 34, 1030–1034 (2014).
Wang, L.-Z. & Li, X.-C. Effect of temperature on incubation and thermal tolerance of the Chinese forest frog. Chin. J. Zool. (2007).
Wang, L. & Li, X.-C. Effects of constant thermal acclimation on thermal tolerance of the Chinese forest frog (Rana chensineniss). Acta Hydrobiol. Sin. 31, 748–750 (2007).
Wang, L.-Z., Li, X.-C. & Sun, T. Preferred temperature, avoidance temperature and lethal temperature of tadpoles of the common giant toad (Bufo gargarizans) and the Chinese forest frog (Rana chensinensis). Chin. J. Zool. 40, 23–27 (2005).
Warburg, M. R. On the water economy of Israel amphibians: The anurans. Comp. Biochem. Physiol. 40, 911–924 (1971).
Warburg, M. R. The water economy of Israel amphibians: The urodeles Triturus vittatus (Jenyns) and Salamandra salamandra (L.). Comp. Biochem. Physiol. 40, 1055–1056, IN11,1057–1063 (1971).
Willhite, C. & Cupp, P. V. Daily rhythms of thermal tolerance in Rana clamitans (Anura: Ranidae) tadpoles. Comp. Biochem. Physiol. 72, 255–257 (1982).
Wu, C.-S. & Kam, Y.-C. Thermal tolerance and thermoregulation by Taiwanese rhacophorid tadpoles (Buergeria japonica) living in geothermal hot springs and streams. Herpetologica 61, 35–46 (2005).
Xu, X. The effect of temperature on body temperature and thermoregulation in different geographic populations of Rana dybowskii. (Harbin Normal University, 2017).
Yandún Vela, M. C. Capacidad de aclimatación en renacuajos de dos especies de anuros: Rhinella marina (Bufonidae) y Gastrotheca riobambae (Hemiphractidae) y su vulnerabilidad al cambio climático. (Pontificia Universidad Católica Del Ecuador, 2017).
Young, V. K. H. & Gifford, M. E. Limited capacity for acclimation of thermal physiology in a salamander. Desmognathus brimleyorum. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 183, 409–418 (2013).
Yu, Z., Dickstein, R., Magee, W. E. & Spotila, J. R. Heat shock response in the salamanders Plethodon jordani and Plethodon cinereus. J. Therm. Biol. 23, 259–265 (1998).
Zheng, R.-Q. & Liu, C.-T. Giant spiny-frog (Paa spinosa) from different populations differ in thermal preference but not in thermal tolerance. Aquat. Ecol. 44, 723–729 (2010).
Zweifel, R. G. Studies on the critical thermal maxima of salamanders. Ecology 38, 64–69 (1957).
Pick, J. L., Nakagawa, S. & Noble, D. W. A. Reproducible, flexible and high-throughput data extraction from primary literature: The metaDigitise r package. Methods Ecol. Evol. 10, 426–431 (2019).
R Core Team. R: A language and environment for statistical computing.
Jetz, W. & Pyron, R. A. The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat. Ecol. Evol. 2, 850–858 (2018).
AmphibiaWeb. https://amphibiaweb.org. University of California, Berkeley, California, USA (2022).
Schwanz, L. E. et al. Best practices for building and curating databases for comparative analyses. J. Exp. Biol. 225, jeb243295 (2022).
Pottier, P. et al. A comprehensive database of amphibian heat tolerance, Zenodo, https://doi.org/10.5281/zenodo.6565454 (2022).
Lajeunesse, M. J. Recovering Missing or Partial Data from Studies: A Survey of Conversions and Imputations for Meta-analysis. in Hanbook of Meta-analysis in Ecology and Evolution 195–206 (Princeton University Press, 2013).
Nakagawa, S., et al. A robust and readily implementable method for the meta-analysis of response ratios with and without missing standard deviations. EcoEvoRxiv, https://doi.org/10.32942/osf.io/7thx9 (2022)
Pottier, P., Burke, S., Drobniak, S. M., Lagisz, M. & Nakagawa, S. Sexual (in)equality? A meta-analysis of sex differences in thermal acclimation capacity across ectotherms. Funct. Ecol. 35, 2663–2678 (2021).
Sunday, J. et al. Thermal tolerance patterns across latitude and elevation. Philos. Trans. R. Soc. B-Biol. Sci. 374, 20190036 (2019).
Truebano, M., Fenner, P., Tills, O., Rundle, S. D. & Rezende, E. L. Thermal strategies vary with life history stage. J. Exp. Biol. 221, jeb171629 (2018).
Rezende, E. L., Tejedo, M. & Santos, M. Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct. Ecol. 25, 111–121 (2011).
Terblanche, J. S., Deere, J. A., Clusella-Trullas, S., Janion, C. & Chown, S. L. Critical thermal limits depend on methodological context. Proc. R. Soc. B-Biol. Sci. 274, 2935–2943 (2007).
Hangartner, S., Sgrò, C. M., Connallon, T. & Booksmythe, I. Sexual dimorphism in phenotypic plasticity and persistence under environmental change: An extension of theory and meta-analysis of current data. Ecol. Lett. (2022).
Wickham, H. et al. Welcome to the tidyverse. J. Open Source Softw. 4, 1686 (2019).
Dunnington, D. & Thorne, B. ggspatial: Spatial Data Framework for ggplot2. R package (2020).
Brownrigg, M. R. Package ‘maps’. R package (2013).
Yu, G., Smith, D. K., Zhu, H., Guan, Y. & Lam, T. T.-Y. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 8, 28–36 (2017).
Xu, S. et al. ggtreeExtra: Compact visualization of richly annotated phylogenetic data. Mol. Biol. Evol. 38, 4039–4042 (2021).
Campitelli, E. ggnewscale: Multiple fill and colour scales in “ggplot2”. R package (2020).
Pedersen, T. L. patchwork: The Composer of Plots. R package (2020).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Acknowledgements
We acknowledge that financial support for this study was provided to PPottier, SB, and PPollo by a UNSW Scientia Doctoral Scholarship. SN is supported by the Australian Research Council (ARC) Discovery Projects (DP200100367). SMD is supported by the ARC Discovery Early Career Award (DE180100202). JOV was funded by the Agencia Nacional de Investigación y Desarrollo of Chile (FONDECYT 3220722) and the National Research, Development and Innovation Office of Hungary (ÉLVONAL KKP-126949, K-116310). TA is supported by ARC Future Fellowship (FT180100354) and the University of Queensland strategic funding. RRYO is supported by the German Center for integrative Research (iDiv) and funded by the German Research Foundation (DFG–FZT 118, 202548816). YY was founded by National Natural Science Foundation of China (32102597). We thank the authors of the original studies we synthesised in our systematic review, without whom this project could not exist.
Author information
Authors and Affiliations
Contributions
Conceptualisation: PPottier, S.B., T.A., S.M.D., S.N. Methodology: PPottier, S.B., T.A., S.M.D., S.N. Software: PPottier, S.M.D., S.N. Formal Analysis: PPottier, S.M.D., S.N. Investigation: PPottier, H.Y.L., R.R.Y.O., PPollo, A.N.R., J.O.V., Y.Y. Data Curation: PPottier. Visualization: PPottier. Writing – Original Draft: PPottier. Writing – Review and Editing: All authors. Project administration: PPottier. Supervision: S.M.D., S.N. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pottier, P., Lin, HY., Oh, R.R.Y. et al. A comprehensive database of amphibian heat tolerance. Sci Data 9, 600 (2022). https://doi.org/10.1038/s41597-022-01704-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-022-01704-9
This article is cited by
-
Heat shock protein 70 in Cynops orientalis: bidirectional thermal regulation and metabolic optimization in amphibian climatic adaptation
BMC Genomics (2026)
-
Global thermal tolerance compilation for freshwater invertebrates and fish
Scientific Data (2025)
-
AmphiTherm: a comprehensive database of amphibian thermal tolerance and preference
Scientific Data (2025)
-
Vulnerability of amphibians to global warming
Nature (2025)
-
Effect of different temperature variations on the physiological state of catfish species: a systematic review
Fish Physiology and Biochemistry (2024)