Abstract
Estuaries are the important interface between the land and sea, providing significant environmental, economic, cultural and social values. However, they face unprecedented pressures including eutrophication, harmful algal blooms, habitat loss, and extreme weather due to climate change. Here we present an open access, quality-controlled water quality dataset collected from twelve diverse estuaries spanning 1000 km along the southeastern Australian coastline. Water depth, temperature and salinity data were collected across two years (2018–2021) capturing drought, wildfire and flood periods, using high accuracy Seabird MicroCAT field sensors located within oyster leases. These fully autonomous instruments collected and transmitted data every 10 minutes before downstream quality checking and uploading onto a public website. Simultaneous, high-resolution, longitudinal environmental data collected across multiple estuaries throughout a range of extreme weather events are exceptionally rare in the Southern Hemisphere, yet provide an invaluable resource for the aquaculture industry, researchers and environmental regulators alike.
Similar content being viewed by others
Background & Summary
Estuaries are complex and diverse coastal ecosystems, being the transition zone between rivers and oceans. They provide vital ecosystem services including environmental (as a critical habitat for many species), cultural (human settlement, trade) and commercial values (fisheries, tourism), however, they are vulnerable to environmental stressors such as coastal development, agriculture, tourism, shipping, sewage disposal, and reduced water quality1,2. Furthermore, because they are intrinsically variable due to rainfall, discharges (or outflows), wind, and tidal ranges, determining the impact of climate change on estuaries is challenging yet understudied, and this knowledge gap hinders our global assessment of climate change3.
There are up to 180 important water bodies which enter the ocean along the eastern seaboard of Australia, the most famous being Sydney Harbour3,4. There is also significant shellfish aquaculture along this coastline, with up to 71 commercial oyster harvest areas in over 30 estuaries, valued around $AUD 70 million, with most of the production focussing on the cultivation of a native species, the Sydney rock oyster (Saccostrea glomerata)5,6. Each of these oyster-growing estuaries has its distinct riverine inputs, tidal cycles, and flushing times4, while concurrently undergoing significant warming7.
Scientists, the aquaculture and fishing industries and government agencies require high quality environmental data to underpin aquatic ecosystem health assessment. These data also assist us to better understand the pressures and threats to aquatic ecosystems. Most of the significant datasets in south-eastern Australia however, have focused either on more oceanic regions (https://portal.aodn.org.au/), are over short time scales, or capture data at a broad frequency, with important temporal small-scale variation overlooked4,7.
The Oyster Industry Transformation Project (2017–2024) aimed to determine the utility of in situ environmental sensor technology, planktonic environmental DNA and sentinel oysters to predict the impact of physico-chemical variables on estuarine water quality. This approach led to improved oyster harvesting and economic benefits by enabling a transition to salinity-based oyster harvest management8. Specifically, we have modelled the causes and consequences of incidences of harmful algal blooms and high levels of pathogenic bacteria in estuaries, leading to more responsive and timely management approaches and crucial data underpinning estuarine health9,10,11.
Moored, high-accuracy sensors were installed in twelve oyster-growing estuaries in 2018, recording real-time, high-resolution temperature, salinity and water depth data. Synchronised with this real-time data collection, weekly biological samples were collected9,10,11 and together, these data were used to develop predictive models for pathogen abundance, harmful algal blooms and oyster growth9,10,11 (https://www.foodagility.com/research/transforming-australian-shellfish-production).
In addition to improving oyster industry and water quality outcomes, the sensor data has been utilised by numerous organisations working on estuarine systems for widely differing applications. Examples include investigations into the link between shark movements and temperature and salinity; habitat suitability modelling to inform restoration of wetlands and oyster reefs12,13; the impacts of flooding on seagrass; hydrodynamic modelling to determine sewage spill impacts; examining soft coral diversity and growth; the validation of satellite water quality monitoring; and determining the entrance status of estuaries.
High-resolution, real-time water quality data collected across multiple and diverse estuaries throughout different climate regimes are rare in the Southern Hemisphere, and constitute valuable data for diverse potential end uses3,4,12,13,14. Here we provide a quality controlled, publicly accessible dataset, providing a unique opportunity to better inform industry, science and management alike.
Methods
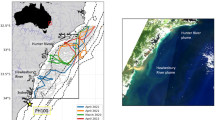
Twelve oyster-growing estuaries spanning 1000 km of southeastern Australian coastline were selected for sensor deployment. These ranged from the Hastings River in the north (subtropical) to Wonboyn Lake in the south (temperate) (Fig. 1a). The majority of these estuaries are wave-dominated, permanently open estuaries, but range from large tide-dominated estuaries (Hawkesbury River, Port Stephens) to significantly smaller lakes (Wapengo and Wonboyn Lakes), and each with varying catchment sizes and flushing rates (Table 1).
(a) Map of southeastern Australia showing estuaries (black dots) where sensors were installed (see Table 1 for estuary details); (b) Seabird sensor located within Wallis Lake estuary.
Located within oyster leases in each of these twelve estuaries, high-resolution water depth (m), temperature (°C), and salinity (ppt) data were collected from 2018 to 2021 using Seabird SBE 37-SM/SMP/SMP-ODO MicroCAT high accuracy CTD sensors (Fig. 1b). Each of these sensors was deployed using a fixed installation, with the inlet 60 cm above the seabed and at least 30 cm below the estimated Lowest Astronomical Tide (LAT). These fully autonomous instruments collected and transmitted data every 10 minutes (24 hday−1) to Microsoft Azure cloud storage before downstream quality checking. Data was then packaged into RO-Crates and uploaded to an Arkisto-based publicly available website (Fig. 2).
Data Records
Approximately 12,000 rows and 1.5 mb of data per site, per month were collected, quality checked and made publicly available at https://salinity.research.uts.edu.au/. This data portal is hosted on Amazon Web service which is a reliable and scalable third-party infrastructure service, the source code is backed up in Github (www.github.com) ensuring recoverability if required, and the metadata are stored in MongoDB (www.mongodb.com) which provides a quick search by NoSQL indexing. The data portal allows for the filtering and downloading of data based on both time (month/year) and location. It also includes metadata corresponding to each data crate and a map showing the sensor location within each estuary. Once downloaded as a csv file, data can be visualised as a time-series for each estuary (Fig. 3).
In addition, the complete dataset is held at the Marine Data Archive (https://doi.org/10.14284/672)15.
Technical Validation
The Seabird SBE 37-SM/SMP/SMP-ODO MicroCAT field sensors were calibrated as per manufacturer’s protocols before each estuary deployment. During operation, cleaning of each sensor occurred on a regular basis to prevent excessive biofouling. For salinity stability testing, water samples were collected within each estuary at the sensor site and cross-checked using salinity method/CSIRO. Briefly, 2–7 sampling times per estuary for water occurred. At these times sampling was carried out during a period in the day when the salinity was expected to be stable (based on readings from the previous days). At this time, a pole sampler equipped with a 200 ml OSIL bottle was used to collect triplicate water samples adjacent to the sensor intake. The bottles were first rinsed then filled allowing for a headspace of approximately 25 cm3. The bottles were then sealed with halobutyl, polymer coated bungs and crimp-caps. These triplicate water samples were collected within 2 minutes of each other, and the time was noted for the first sample collected. Back on shore, the salinity reading 10 mins either side of the sampling time was noted, and the salinity interpolated across these values was also noted.
Water samples were then sent to the Commonwealth Scientific and Industrial Research Organisation (CSIRO) for analytical testing. Salinity measurements were performed on a Guildline Autosal 8400B instrument operated in accordance with its technical manual. The measured values were recorded with an OSIL data logger. Practical salinity (S) is defined in terms of the ratio (K15) of the electrical conductivity measured at 15 °C 1 atm of seawater to that of a potassium chloride (KCl) solution of mass fraction 32.4356 × 10−3. Salinity is determined by measuring the electrical conductivity ratio of a given sample, which is converted to practical salinity using a mathematical equation. The conductivity ratio varies with temperature so the salinometer contains a temperature-controlled bath. The sample is pumped through a heat exchanger inside the temperature-controlled bath to raise it to the desired temperature. It then flows into the instrument’s conductivity cell, which is also kept at a constant temperature. Inside the conductivity cell there are four coiled electrodes. The two outer coils are used as potential leads while the inner coils are current leads. The conductivity circuit measures the resistance of the sample by maintaining a precise voltage across the cell and measuring the voltage across a stable reference resistor arranged in series with the current leads of the cell electrodes. The instrument output is displayed as a double conductivity ratio and is calibrated to IAPSO recognised seawater standards. Before each lot of sample measurements, the Autosal is calibrated with standard seawater (OSIL, IAPSO) of known K15 ratio. A new bottle of OSIL standard is used for each calibration. The frequency of calibration is at least one per run. To measure, the Autosal cell is flushed three times with the sample and then measured after the fourth and fifth flush. The OSIL data logger software captures the conductivity ratio and calculates the practical salinity.
If the triplicate salinity results for a sensor site were relatively similar they were averaged, and that value then compared to the sensor reading. If one result was widely different from the other two, the sensor trace was re-examined. If the variation could not be explained, the anomalous sample was rejected (potentially contaminated), and the average of the other two samples calculated and compared to the sensor data. In all cases, the sensor data measured salinity within the acceptable range of 0.5 ppt when compared to the laboratory test result (Supplementary Table 1).
Code availability
The source code for the data portal is freely available at https://github.com/UTS-eResearch/oni-express and the data generation available at https://github.com/UTS-eResearch/seafood-collection.
References
Booi, S., Mishi, S. & Andersen, O. Ecosystem Services: A Systematic Review of Provisioning and Cultural Ecosystem Services in Estuaries. Sustainability 14, 7252 (2022).
Martin, C. L., Momtaz, S., Gaston, T. & Moltschaniwskyj, N. A. Estuarine cultural ecosystem services valued by local people in New South Wales, Australia, and attributes important for continued supply. Ocean and Coastal Management 190, 105160 (2020).
Biguino, B., Haigh, I. D., Dias, J. M. & Brito, A. C. Climate change in estuarine systems: Patterns and gaps using a meta-analysis approach. Science of The Total Environment 858, 159742 (2023).
Roy, P. S. et al. Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53, 351–384 (2001).
NSW Department of Primary Industries. Aquaculture Production Report 2021-2022. 19 (2023).
BDO EconSearch. Economic contribution of aquaculture to New South Wales. A Report for NSW Department of Primary Industries. 37 (2023).
Scanes, E., Scanes, P. R. & Ross, P. M. Climate change rapidly warms and acidifies Australian estuaries. Nature Communications 11, 1803, https://doi.org/10.1038/s41467-020-15550-z (2020).
Wicks, S. & Farrell, H. Drought and above average rainfall impacts on the net returns to NSW oyster production – using real-time sensors. NSW DPI https://www.foodauthority.nsw.gov.au/sites/default/files/2023-04/Oyster%20Realtime%20Sensor%20CBA%20Report_13142023%20-%20FINAL.pdf (2023).
Ajani, P. A. et al. Mapping the development of a Dinophysis bloom in a shellfish aquaculture area using a novel molecular qPCR assay. Harmful Algae 116, https://doi.org/10.1016/j.hal.2022.102253 (2022).
Ajani, P. A. et al. Using qPCR and high-resolution sensor data to model a multi-species Pseudo-nitzschia (Bacillariophyceae) bloom in southeastern Australia. Harmful Algae 108, 102095, https://doi.org/10.1016/j.hal.2021.102095 (2021).
McLennan, K., Ruvindy, R., Ostrowski, M. & Murray, S. Assessing the Use of Molecular Barcoding and qPCR for Investigating the Ecology of Prorocentrum minimum (Dinophyceae), a Harmful Algal Species. Microorganisms 9, 9030510 (2021).
Howie, A. H. & Bishop, M. J. Contemporary oyster reef restoration: Responding to a changing world. Frontiers in Ecology and Evolution 9, 689915 (2021).
Roper, T. et al. Assessing the condition of estuaries and coastal lake ecosystems in NSW. Technical report (13 May 2014), NSW State of the Catchments 2010. 231 (2011).
Hughes, M. G., Rogers, K. & Wen, L. Saline wetland extents and tidal inundation regimes on a micro-tidal coast, New South Wales, Australia. Estuarine, Coastal and Shelf Science 227, 106297 (2019).
Murray, S. & Ajani, P. High-resolution temperature, salinity and depth data from southeastern Australian estuaries. Marine Data Archive. https://doi.org/10.14284/672 (2024).
Acknowledgements
This project has been funded under the Bushfire Local Economic Recovery Fund, co-funded by the Australian and NSW Governments in association with the Food Agility CRC and the NSW Farmer’s Association. The Food Agility CRC Ltd is funded under the Commonwealth Government CRC Program. The CRC Program supports industry-led collaborations between industry, researchers and the community. The Department of Primary Industries, Hunter Local Land Services and the University of Technology Sydney also provided project funding. The project team would like to acknowledge the invaluable assistance of staff from The Yield Technology Solutions for providing water salinity and temperature data and NSW oyster farmers for their assistance with site access and sensor maintenance. We also acknowledge the eResearch team at UTS, particularly Simon Kruik, Michael Lynch, Xixin He and Bala Naidu for the data portal set up and the data submission to Marine Data Archive, and the hydrochemistry team at CSIRO Hobart for salinity testing. We thank Javier Pérez Burillo for the map.
Author information
Authors and Affiliations
Contributions
P.A. contributed to study conception and design, project coordination and data analysis; M.D. contributed to study conception and design, and data analysis; H.F. contributed to study conception and design, and data analysis; W.O’C. contributed to study conception and design, and data analysis; M.T. provided technical assistance for data collection and analysis; A.V. provided technical assistance for data collection and analysis; A.Z. contributed to study conception and design; B.H. contributed to study conception and design; S.M. contributed to study conception and design and project coordination. All authors contributed to interpretation of results and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ajani, P., Dove, M., Farrell, H. et al. High-resolution temperature, salinity and depth data from southeastern Australian estuaries, 2018–2021. Sci Data 11, 968 (2024). https://doi.org/10.1038/s41597-024-03828-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-024-03828-6