Abstract
How emerging adulthood, a period particularly susceptible to the onset of mental disorders, responds to acute aerobic exercise effects on mood and brain remains an open question. A challenge in addressing this question is the scarcity of open data derived from scientific intervention paradigms. We recruited 83 healthy volunteers aged 17 to 24, who were randomly assigned to either an exercise group or a control group. The exercise group performed 30-minutes aerobic exercise with moderate intensity on a cycle ergometer, while the control group read neutral textual material without any physical activity. All participants received structural MRI scans before their intervention periods and completed mood scales and resting-state functional MRI scans before and after their intervention periods. Their heart rates were also monitored during the intervention. The dataset represents the first open resource for probing the acute aerobic exercise effects on brain and mind, containing physical, mental, and brain measurements. It has been released as part of the Chinese Color Nest Community in the Science Data Bank.
Similar content being viewed by others
Background & Summary
Emerging adulthood represents a critical period in which individuals navigate the transition from adolescence to independent adulthood, often encountering mental health challenges along the way. An extensive investigation with 61,880 adolescents and adults revealed that the incidence of mood disorders increased with age among adolescents aged 12 to 17 years, while it decreased among adults aged 18 to 25 years1. A comprehensive meta-analysis including 192 epidemiological studies showed that the median age of onset for all psychiatric disorders was 18 years of age2.Notably, this phase is characterized by relative independence from social roles and normative expectations, marking a departure from the dependency of childhood and adolescence without fully embracing the responsibilities of adulthood3. For many new college students, the transition to university life can be particularly stressful, heightening vulnerability to stress and depression.4. Therefore, exploring effective strategies to improve emotional health among emerging adults is of paramount importance.
Structured exercise has been proven to be an effective intervention for improving mental health in this demographic5,6. Research has consistently shown that exercise can mitigate the risk of various psychiatric disorders7,8 and alleviate symptoms of depression9, anxiety10, and bipolar disorder11. Moreover, exercise is increasingly recommended as a complementary and alternative medicine due to its safety and lack of side effects associated with pharmacological treatments12. Based on accumulative evidence in this field, international guidelines for treating mood or emotional disorders recommend integrating physical activity and exercise into health care and intervention13,14,15. Notably, the effectiveness of exercise on mood appears to be dose-dependent16,17, with moderate-intensity aerobic exercise yielding the most significant improvements18,19. Recent studies suggest that just 20 minutes of exercise can produce substantial benefits20, with 30 minutes of moderate-intensity exercise being commonly recommended18,21.
Despite the documented benefits of exercise on emotional well-being, the underlying neural mechanisms remain poorly understood. Neuroimaging studies have provided valuable insights, revealing correlations between exercise and changes in brain connectivity and activity, particularly in regions associated with emotional processing22,23.Notably, exercise-induced enhancements in functional connectivity between brain regions have been linked to reductions in negative affect21,24. Overall, these findings suggest that exercise may have a profound impact on emotional well-being through complex neural mechanisms.
This dataset offers valuable insights into the effects of acute exercise on mood and brain activity during emerging adulthood. Using a randomized controlled design, participants engaged in 30 minutes of moderate-intensity aerobic exercise, with mood assessments and Resting-state functional magnetic resonance imaging (rfMRI) scans conducted before and after the intervention. The dataset’s comprehensive nature, encompassing physical, mental, and brain data, along with its large sample size, positions it as a valuable resource for future research in this field.
Methods
Participants
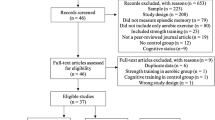
A total of eighty-three healthy, right-handed volunteers aged 17 to 24 (mean age = 18.82, SD = 1.31) were recruited from a local university (36 females and 47 males). Among them, 76 individuals possess comprehensive neuroimaging data. Participants were excluded if they met any of the following criteria: (1) being ambidextrous or left-handed, (2) having neurological or psychiatric disorders, (3) suffering from severe somatic disorders affecting exercise, (4) taking any medications within a month or having a history of substance abuse, (5) having intact cognitive function, or (6) having MRI contraindications. All participants abstained from alcohol consumption and physical exercise 48 hours prior to the test. The study received approval from the ethics committee of the Institute of Psychology, Chinese Academy of Sciences (approval number: H15038). Written informed consent was obtained from all participants, and they all volunteered to participate in data collection and further data sharing. Although one participant was 17 years old, he/she was considered capable of providing informed consent under the supervision of the university’s ethics committee, as he/she was already enrolled as a university student.
Image acquisition
Each participant underwent MRI scans using a 3.0 Tesla scanner (MAGNETOM TrioTim, Siemens, Erlangen, Germany) with a 12-channel head matrix coil. The rs-fMRI sequences were acquired using an echo-planar imaging (EPI) sequence with the following parameters: number of slices = 33, field of view (FOV) = 200 × 200 mm2, slice thickness = 5.0 mm, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, matrix = 64 × 64, voxel size = 3.1 × 3.1 × 5.0 mm3, and interleaved slice acquisition order. High-resolution T1-weighted structural images were acquired using a magnetization prepared rapid gradient echo (MPRAGE) three-dimensional sequence with the following parameters: FOV = 256 × 256 mm2, TR = 2530 ms, inversion time = 1100 ms, TE = 3.45 ms, FA = 7°, slice thickness = 1.0 mm, matrix = 256 × 256, and voxel size = 1.0 × 1.0 × 1.0 mm3.
Quality assurance
All 76 participants were included in the first-step quality assessments of the neuroimaging data. Their anatomical T1 sMRI images were visually inspected to ensure no substantial head motion and structural abnormalities. Fourteen participants were excluded for excessive head motion exceeding a mean framewise displacement (FD) threshold of 0.2 mm (Fig. 1). In the second-step quality assessments, the T1 sMRI and rfMRI image quality of this datasets were accessed using the MRIQC toolkit (https://github.com/poldracklab/mriqc), which incorporated a series of quantitative metrics as in the following list. More details of these metrics can be found in the previous work25,26.
Behavioral measurements
Demographic Information and Physical activity rating scale (PARS-3)
Essential demographic details, including gender, age, academic year, height, and weight, were collected. The 3-item Chinese version27 of PARS-328 was used to assess participants’ physical activity level based on frequency, duration, and intensity of their physical activity using a 5-point scale. The overall physical activity score was the product of frequency, duration (minus 1) and intensity. The test–retest reliability of PARS-3 was 0.8227.
Positive and Negative Affect Schedule (PANAS)
We applied the Chinese version29 of Positive and Negative Affect Schedule (PANAS)30 to measures both positive affect (PA) and negative affect (NA). The internal consistency reliabilities, as measured by Cronbach’s coefficient α, for the reference sample are highly acceptable, with PA of 0.86 ∼ 0.90 and NA of 0.84 ∼ 0.87.The PANAS consists of 20 items, each of which is an adjectival word describing an acute emotional state, with 10 adjectives describing positive affect and 10 describing negative affect. Answers are rated on a 5-point Likert scale ranging from 1 (very mild) to 5 (very strong).
Abbreviated profile of mood state (A-POMS)
The 40-item Chinese version31 of the abbreviated profile of mood state (A-POMS)32 was used to measure mood states before and after exercise.The A-POMS was developed from the POMS-SF and has acceptable psychometric properties for use in a sport setting. The scale consists of seven subscales, including “tension”, “depression”, “fatigue”, “vigor”, “confusion”, “anger”, and “esteem-related affect”, which can be summed to obtain a “Total Mood Disturbance” (TMD) score. The reliability of the subscales of A-POMS ranged from 0.62 to 0.82, with a mean of 0.71. All ratings were measured on a 5-point Likert scale ranging from 0 (Not at all) to 4 (Extremely).
State-Trait Anxiety Inventory (STAI)
We employed the Chinese version33 of the State-Trait Anxiety Inventory (STAI)34 to measure immediate experiences or feelings before and after the intervention or control condition from all participants. The state anxiety subscale, which consists of 20 items rated on a 4-point Likert scale ranging from 1 (almost never) to 4 (nearly always), demonstrated high test-retest reliability with a coefficient of 0.88.
Exercise intensity monitoring
Exercise intensity monitoring
The heart rate (HR) of each participant during exercise and recovery was recorded by a Polar heart rate monitor (Sport Tester PE 3000, Polar Electro Oy, Finland) at two-minute intervals. Age predicted maximum heart rate (APMHR = 220-age) was calculated for each participant based on their age to monitor their exercise intensity.
Borg Rating of Perceived Exertion (RPE)
The Borg Rating of Perceived Exertion (RPE)35 is a subjective measure of exercise intensity that assesses how hard an individual feels like they are working during exercise. The RPE scale ranges from 6 to 20, with 6 indicating “no effort at all” and 20 indicating “maximum effort.” For example, a rating of 13 on the RPE scale corresponds to a heart rate of 130 beats per minute, which is typically considered moderate intensity exercise.
Procedure
Participants completed all experimental tasks within a single half-day visit to the laboratory (Fig. 2). Prior to the study, participants were screened against specific inclusion criteria and provided informed consent. Following screening and informed consent, participants completed the behavioral measurements. Random assignment allocated participants to either the acute exercise group (AG) or the control group (CG). The study comprised three sessions: pre-test, intervention or control condition, and post-test. Both the pre-test and post-test included emotion tests and MRI scans, interspersed with a 30-minute exercise intervention or control condition.
Experimental design. The study comprised pre-test, intervention or control condition, and post-test sessions. Pre-test: Participants completed a behavioral measurement that included demographic information, physical activity rating scale, and emotional measurement, followed by an MRI scan that consisted of two 10-minute phases of resting-state functional magnetic resonance imaging (rest1 and rest2) and a T1-weighted structural MRI. The intervention condition was a 30-minute moderate-intensity aerobic exercise on a cycle ergometer, during which heart rate and Borg Rating of Perceived Exertion were monitored. Post-test: Participants repeated the emotional measurements and the 10-minute rfMRI scan (rest3).
After successfully completing the MRI safety questionnaire, each participant was sequentially scanned as follows: (1) Localizer, (2) a 10-minute rfMRI session, labeled as rest1, (3) a 5-minute relaxation interval to allow participants to return to a relaxed state, (4) another 10-minute rfMRI session (rest2), and (5) a 3D T1-weighted structural MRI (sMRI). The purpose of acquiring two separate resting-state scans with a relaxation interval was to enhance the reliability of the measurements, reduce motion artifacts, and allow participants to relax between scans. Subsequent to the intervention, participants underwent a post-test scanning sequence, consisting of the Localizer followed by an rfMRI session, referred to as rest3 for this stage.
The acute exercise interventions were conducted using a MONARK 834 cycle ergometer (MONARK, Varberg, Sweden). The exercise protocol involved a 5-minute warm-up, a 20 minutes of moderate-intensity aerobic exercise, and a five-minute cool-down. The warm-up phase commenced at a workload equivalent to 60% of the participants’ APMHR, approximately 25 W power output and 50 rpm pedaling rate, which was gradually increased until the exercise intensity reached 60–69% of the APMHR, with an average 77 ± 4 rpm pedaling rate. The HR, RPE scales, power output (in watts), and revolutions per minute were recorded every 2 minutes during the intervention. After the exercise intervention, participants were required to rest until their HR returned to baseline levels, and only then proceed with the post-test session MRI scan, which had to be completed within 10 minutes. In the control group, participants were instructed to read neutral textual material in a noise-free space for 30 minutes while monitoring their heart rate. Usage of cell phones was prohibited during this period.
Data Records
The dataset is available at Science Databank (https://cstr.cn/31253.11.sciencedb.1422236). To access the dataset, users must apply using the Data Usage Agreement (DUA) available at https://doi.org/10.57760/sciencedb.o00133.0002037. The DUA outlines the terms and conditions for data access, and users must upload the signed agreement with their application to request access to the dataset. The database comprises resting-state functional magnetic resonance imaging (rs-fMRI) data from 76 right-handed Chinese healthy volunteers. As proponents of open science, we are pleased to announce the release of this database to support research and educational activities in the fields of human brain mapping and cultural association studies. Following anonymization of MRI data through a face masking protocol derived from the CN200 brain template38. Subsequently, we meticulously structured the data in accordance with the Brain Imaging Data Structure (BIDS: https://bids.neuroimaging.io)39 framework. Additionally, we utilized the MRIQC toolkit to ensure the stringent quality control of both structural and functional images. This toolkit has been instrumental in producing detailed reports on data quality. All data, formatted in compliance with BIDS standards, along with accompanying quality control reports, are openly accessible through the Chinese Color Nest Community at Science Data Bank (SciDB: https://ccnp.scidb.cn) and made publicly available through the Interdisciplinary Brain Database for In-vivo Population Imaging (ID-BRAIN) at the National Basic Science Data Center. This effort reflects our dedication to promote transparency and facilitate scientific progress through the sharing of data.
The “DATA” folder contains 62 subfolders labeled with participant ID numbers (e.g., sub-002). Each participant folder contains subfolders labeled “func,” which include rs-fMRI images (.nii.gz), and “anat,” which include defaced T1-weighted structural images (.nii.gz). Additionally, the “QC” folder contains quality control reports generated by the MRIQC toolkit for each participant. All QC results were integrated into webpages and linked in an overview page on group analysis.
Technical Validation
To demonstrate the utility of this released MRI data, we derived two sets of brain imaging metrics using the Connectome Computation System (CCS)40 for both structural and functional characteristics. In addition to the quality control metrics mentioned earlier, a set of structural and functional derivatives were calculated for the datasets included in this dataset:
-
Regional Homogeneity (ReHo)41: Measures the local brain functional homogeneity by assessing the synchronicity of a voxel’s time series and that of its nearest neighbors based on Kendall’s coefficient of concordance.
-
Voxel-Mirrored Homotopic Connectivity (VMHC)42: Quantifies the functional connectivity between geometrically symmetric, inter-hemispheric voxels.
-
Amplitude of low-frequency fluctuations (ALFF)43:Estimates the total power in the low frequency range (0.01–0.1 Hz) of an fMRI image, normalized by the total power across all frequencies.
All preprocessing steps were implemented using CCS with the most recent updates40,44 (https://github.com/zuoxinian/CCS). This pipeline integrates multiple analytical software packages to achieve imaging processing of multi-modal MRI data. Considering the advantages of surface-based functional brain mapping41, we reconstructed cortical surface models to generate NIFTI file for structural and functional metrics on the cortical surface. The structural image went through the following preprocessing steps: (1) spatially adaptive non-local means denoising, (2) rough inhomogeneity correction, (3) spatial normalization into the MNI standard brain space, (4) inhomogeneity correction, (5) intensity normalization, (6) brain extraction by non-local intracranial cavity extraction (NICE), and (7) gray and white matter segmentation, surface reconstruction. The rfMRI image preprocessing included (1) dropping off the first 5 EPI volumes, (2) removing and interpolating temporal spikes, (3) correcting acquisition timing among image slices and head motion among image volumes, (4) normalizing the 4D global mean intensity to 10,000, (5) regressing out head motion artifacts and other spurious noise by using ICA-AROMA, and (6) removing linear and quadratic trends from the rfMRI signals to mitigate the scanner-related influences40.
The NIFTI images were converted into the GIFTI format using the Ciftify toolbox45. Structural data were preprocessed using the custom pipeline to generate highly refined cortical surface meshes which were spatially normalized to MNI space and re-sampled to have 32k vertices. Surface-mapped BOLD signals were brought into register across participants using a multi-modal surface matching algorithm to the fsaverage_LR32 space and vectorized to CIFTI format, mapping each surface vertex to an index in a vector by Ciftify toolbox. A group-level surface mask was established by including every vertex showing rfMRI signals across the cortex.
This technical validation process ensures the reliability and reproducibility of the released MRI data and derivatives46, enabling robust research outcomes in the field of human brain mapping.
Usage Notes
This dataset is intended for researchers interested in exploring the effects of acute aerobic exercise on brain activity in emerging adulthood. It includes resting-state functional magnetic resonance imaging (rs-fMRI) data from 76 right-handed Chinese healthy volunteers, along with demographic, behavioral and physiological measures of mood and exercise intensity. The data was collected as part of a randomized controlled trial investigating the effects of acute aerobic exercise on brain function and mood in emerging adulthood. It is available for reuse by other researchers interested in this topic. Figure 3 depicts the effects of acute aerobic exercise on spontaneous cortical activity in college students.
Acute aerobic exercise effects on spontaneous cortical activity in college students. Three metrics of human cortical spontaneous activity including ALFF, ReHo, and VMHC were mapped vertex-wise onto the cortical surfaces for the Pre-test (the mean of rest1 and rest2) and Post-test (rest3) conditions. Differences between Post-test and Pre-test were also rendered onto the surfaces.
A portion of this dataset has been utilized in previous publications. In a smaller sample, we observed that the enhancement in negative affect following moderate-intensity acute aerobic exercise might be linked with improved functional connectivity between the orbitofrontal lobe and the amygdala21. This dataset lends itself to various downstream analyses, including:
Prediction
Exploring whether the baseline brain structure and activity can predict the post-exercise changes in mood and heart rate, as well as the exercise-induced changes in brain function. This can be done by using regression models or machine learning algorithms to test the associations between the baseline and post-test variables. For instance, supervised learning algorithms such as support vector machines or random forests could be utilized. The dataset would need to be divided into a training set for model training and a test set for performance evaluation. Prediction accuracy can be assessed using cross-validation or other suitable metrics. The results could offer insights into individual differences and variability in the effects of exercise.
Baseline correlation analysis
Examining if the baseline brain structure or activity is correlated with the physical activity level and the baseline heart rate. This can help to identify the potential factors that influence the baseline mood and brain activity. This can be done by using correlation analysis or other statistical methods to measure the strength and direction of the relationships between the variables. Regression models could also be used to control for potential confounding variables. The results could reveal the baseline characteristics and profiles of the participants, and provide insights into the relationships between physical activity, heart rate, brain structure and function.
Brain function analysis
Correlating exercise-induced changes in resting-state brain function with changes in mood and heart rate using correlation analysis or multivariate regression models. This analysis could enhance understanding of the mechanisms and effects of acute exercise on emotion. Dynamic functional connectivity analysis can also be performed to examine the temporal changes in brain network interactions. This would involve calculating the functional connectivity between different brain regions at different time points and looking for patterns of change over time. This could provide insights into how brain networks change over time in response to acute exercise.
Integration
Integrating this dataset with other datasets that share similar variables or methods, such as other datasets of exercise that measure mood or brain activity in different populations or contexts. This could help to increase the sample size and diversity of the dataset, as well as to test for potential confounding factors or moderators. Integration would require ensuring variables are measured compatibly. Meta-analysis or pooled analysis techniques could be used to combine the results from different datasets. It would be necessary to check for heterogeneity between the datasets and use random-effects models if necessary.
Comparison
Comparing this dataset with others with different characteristics or objectives, such as different exercise interventions (e.g., resistance training, yoga), different duration of exercise intervention, or different ethnic or cultural. This might evaluate findings’ validity, reliability, and identify bias. To compare this dataset with other datasets that have different characteristics or objectives, appropriate statistical tests would need to be used. Appropriate statistical tests, such as t-tests or chi-square tests, would be used, ensuring data meet test assumptions.
Please note this dataset is based on a single study with a relatively small sample size (n = 76), so the results may not be generalizable to other populations or contexts. This dataset is available at Science Databank36. Users must apply to access the data by completing the Data Usage Agreement at https://doi.org/10.57760/sciencedb.o00133.0002037 and uploading it with their application. Access to the dataset is granted after approval of the DUA. To access the dataset, investigators must complete the Chinese Color Nest Project Data Access Request (DUA) located at: https://doi.org/10.57760/sciencedb.o00133.00020. Dataset users should acknowledge original authors’ contributions, cite the dataset correctly (available instructions on the Science Data Bank website: https://cstr.cn/31253.11.sciencedb.14222), and contact for additional data sharing and cooperation. We aim for this dataset to advance understanding of exercise behavior’s association with the human brain.
Code availability
All the scripts and brain templates involved for processing is publicly available as part of the CCS updates44 and can be found at GitHub (https://github.com/zuoxinian/CCS/tree/master).
References
Twenge, J. M., Cooper, A. B., Joiner, T. E., Duffy, M. E. & Binau, S. G. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005–2017. Journal of abnormal psychology 128, 185 (2019).
Solmi, M. et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Molecular psychiatry 27, 281–295 (2022).
Arnett, J. J. Emerging adulthood: A theory of development from the late teens through the twenties. American psychologist 55, 469 (2000).
Dyson, R. & Renk, K. Freshmen adaptation to university life: Depressive symptoms, stress, and coping. Journal of clinical psychology 62, 1231–1244 (2006).
Collins, S. et al. A longitudinal study of lifestyle behaviours in emerging adulthood and risk for symptoms of depression, anxiety, and stress. Journal of affective disorders (2023).
Kwan, M. Y., Ceccacci, A., Paolucci, N. & Rebar, A. Physical activity and internalizing symptoms during the transition from adolescence to emerging adulthood: a systematic review of prospective and longitudinal studies. Adolescent research review 6, 75–89 (2021).
Larun, L., Nordheim, L. V., Ekeland, E., Hagen, K. B. & Heian, F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane database of systematic reviews (2006).
Mayer, J. S. et al. Bright light therapy versus physical exercise to prevent co-morbid depression and obesity in adolescents and young adults with attention-deficit/hyperactivity disorder: study protocol for a randomized controlled trial. Trials 19, 1–19 (2018).
Nasstasia, Y. et al. Differential treatment effects of an integrated motivational interviewing and exercise intervention on depressive symptom profiles and associated factors: a randomised controlled cross-over trial among youth with major depression. Journal of affective disorders 259, 413–423 (2019).
Stubbs, B. et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry research 249, 102–108 (2017).
Subramaniapillai, M. et al. Characterizing exercise-induced feelings after one bout of exercise among adolescents with and without bipolar disorder. Journal of affective disorders 190, 467–473 (2016).
Imboden, C. et al. Aerobic exercise or stretching as add-on to inpatient treatment of depression: Similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. Journal of affective disorders 276, 866–876 (2020).
Carneiro, L. F., Mota, M. P., Schuch, F., Deslandes, A. & Vasconcelos-Raposo, J. Portuguese and brazilian guidelines for the treatment of depression: exercise as medicine. Brazilian Journal of Psychiatry 40, 210–211 (2017).
Stubbs, B. et al. Epa guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and position statement from the european psychiatric association (epa), supported by the international organization of physical therapists in mental health (ioptmh). European Psychiatry 54, 124–144 (2018).
Hallgren, M. et al. Treatment guidelines for depression: greater emphasis on physical activity is needed. European Psychiatry 40, 1–3 (2017).
Dunn, A. L., Trivedi, M. H., Kampert, J. B., Clark, C. G. & Chambliss, H. O. Exercise treatment for depression: efficacy and dose response. American journal of preventive medicine 28, 1–8 (2005).
Wipfli, B. M., Rethorst, C. D. & Landers, D. M. The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose–response analysis. Journal of Sport and Exercise Psychology 30, 392–410 (2008).
Tate, A. K. & Petruzzello, S. J. Varying the intensity of acute exercise: implications for changes in affect. The Journal of sports medicine and physical fitness 35, 295–302 (1995).
Ligeza, T. S., Nowak, I., Maciejczyk, M., Szygula, Z. & Wyczesany, M. Acute aerobic exercise enhances cortical connectivity between structures involved in shaping mood and improves self-reported mood: An eeg effective-connectivity study in young male adults. International Journal of Psychophysiology 162, 22–33 (2021).
Berger, B. G. & Motl, R. W. Exercise and mood: A selective review and synthesis of research employing the profile of mood states. Journal of applied sport psychology 12, 69–92 (2000).
Ge, L.-K., Hu, Z., Wang, W., Siu, P. M. & Wei, G.-X. Aerobic exercise decreases negative affect by modulating orbitofrontal-amygdala connectivity in adolescents. Life 11, 577 (2021).
Schmitt, A. et al. Affective modulation after high-intensity exercise is associated with prolonged amygdalar-insular functional connectivity increase. Neural Plasticity 2020 (2020).
Tozzi, L. et al. Longitudinal functional connectivity changes correlate with mood improvement after regular exercise in a dose-dependent fashion. European Journal of Neuroscience 43, 1089–1096 (2016).
Chen, Y.-C., Chen, C., Martínez, R. M., Etnier, J. L. & Cheng, Y. Habitual physical activity mediates the acute exercise-induced modulation of anxiety-related amygdala functional connectivity. Scientific reports 9, 1–12 (2019).
Esteban, O. et al. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One 12, e0184661 (2017).
Esteban, O. et al. Crowdsourced MRI quality metrics and expert quality annotations for training of humans and machines. Scientific Data 6, 30 (2019).
Liang, D. Stress level of college students and its relationship with physical exercise. Chin. Ment. Health J 8 (1994).
Hashimoto, K. Stress, exercise and quality of life proceedings. In Beijing Asian Games a Scientific Congress (Foshan: Zhejiang Zhimei Printing & Packaging Co. Ltd), 16–20 (1990).
Huang, L., Yang, T. & Li, Z. Applicability of the positive and negative affect scale in chinese. Chinese Mental Health Journal (2003).
Watson, D., Clark, L. A. & Tellegen, A. Development and validation of brief measures of positive and negative affect: the panas scales. J Pers Soc Psychol 54, 1063–1070 (1988).
Zhu, B. Brief introduction of poms scale and its model for china. Journal of Tianjin Institute of Physical Education 10, 35–37 (1995).
Grove, J. R. & Prapavessis, H. Preliminary evidence for the reliability and validity of an abbreviated profile of mood states. International Journal of Sport Psychology 23, 93–109 (1992).
Li, W. & Qian, M. Revision of the state-trait anxiety inventory with sample of chinese college students. Acta Scientiarum Naturalium Universitatis Pekinensis 31, 108–114 (1995).
Spielberger, C. D. Manual for the state-trait anxiety inventory. Self Evaluation Questionnaire (1970).
Borg, G. Borg’s perceived exertion and pain scales. (Human kinetics, 1998).
Ge, L.-K., Gao, P., Wei, G.-X. & Zuo, X.-N. Brain Imaging Dataset in Exercise-induced Behavioral Change among Chinese Sample, https://doi.org/10.57760/sciencedb.14222 (2023).
Gao, P. & Zuo, X.-N. Chinese Color Nest Project Data Access Request, https://doi.org/10.57760/sciencedb.o00133.00020 (2024).
Gao, P. et al. A chinese multi-modal neuroimaging data release for increasing diversity of human brain mapping. Scientific Data 9, 286 (2022).
Gorgolewski, K. et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Scientific Data 3, 160044 (2016).
Xu, T., Yang, Z., Jiang, L. L., Xing, X. X. & Zuo, X. N. A Connectome Computation System for discovery science of brain. Science Bulletin 60, 86–95 (2015).
Zuo, X. N. et al. Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. Neuroimage 65, 374–386 (2013).
Zuo, X. N. et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. Journal of Neuroscience 30, 15034–15043 (2010).
Zuo, X. N. et al. The oscillating brain: Complex and reliable. Neuroimage 49, 1432–1445 (2010).
Xing, X. X., Xu, T., Jiang, C., Wang, Y. S. & Zuo, X. N. Connectome Computation System: 2015–2021 updates. Science Bulletin 67, 448–451 (2022).
Dickie, E. W. et al. Ciftify: A framework for surface-based analysis of legacy MR acquisitions. Neuroimage 197, 818–826 (2019).
Zuo, X. N. et al. An open science resource for establishing reliability and reproducibility in functional connectomics. Scientific Data 1, 1–13 (2014).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31671163, 32471133) and STI 2030—Major Projects 2021ZD0200500. The team receives funding support from the National Basic Science Data Center “Interdisciplinary Brain Database for In-vivo Population Imaging” (ID-BRAIN: NBSDC-DB-15), the Key-Area Research and Development Program of Guangdong Province (2019B030335001), the Start-up Funds for Leading Talents at Beijing Normal University, and the Open Research Fund of the Computer Network Information Center, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Gao-Xia Wei and Xi-Nian Zuo designed the study. Li-Kun Ge, Peng Gao, Da Chang and Yinshan Wang did statistical analysis. Gao-Xia Wei and Li-Kun Ge were responsible for data collection. All authors were involved in writing the manuscript. Gao-Xia Wei, Yinshan Wang and Xi-Nian Zuo reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ge, LK., Gao, P., Chang, D. et al. An open data for imaging acute aerobic exercise effects on brain and mind in emerging adulthood. Sci Data 11, 1422 (2024). https://doi.org/10.1038/s41597-024-04270-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-024-04270-4