Abstract
The 626M24 dataset created during this project contains validated experimental transitions and empirical rovibrational energy levels for the parent carbon dioxide isotopologue, 12C16O2 (in a shorthand notation, 626). Validation of the measured transitions and determination of the empirical energy levels is based on a compiled and carefully checked dataset of experimental rovibrational transitions collected from 143 literature sources. The 44 828 measured lines collected, in the wavenumber range of 42.9 – 14 076 cm−1, describe 22 218 unique transitions. Inversion of the experimental information yields 8268 empirical rovibrational energy levels for 626, with uncertainty estimates compliant with the experimental uncertainties of the transitions. Comparison with the Carbon Dioxide Spectroscopic Databank (CDSD-296), NASA Ames-2021, and HITRAN2020 line catalogues shows generally good agreement and suggests some possible improvements to these databases. The 626M24 dataset and an extended line list, called 626M24LL, built upon it and containing 285 503 line positions, are deposited in an OSF (Open Science Framework) repository.
Similar content being viewed by others
Background & Summary
The triatomic carbon dioxide molecule, CO2, contains two terminal oxygen and a single central carbon atom, and in the electronic ground state it has a linear equilibrium structure. Studying the internal motions and related rovibrational spectra of carbon dioxide at relatively low temperatures, so that none of the excited electronic states need to be taken into account, is relevant to many fields of science and technology.
The variation in the CO2 content of the atmosphere of Earth over time is one of its important characteristics1. Detailed understanding of CO2 spectroscopic features2,3, in particular the line center positions, both with and without collisional effects, helps to establish the total amount and the distribution of this molecule in the atmosphere of Earth. These data also help to understand the effect of CO2 on a number of environmental issues, such as climate change and the radiative balance of our atmosphere. The industrial revolution has had a significant impact on climate change; during the last century, the well-documented increase of CO2’s concentration in the atmosphere of the Earth is predominantly due to human activity4. Approximately 98.45 % of carbon dioxide molecules in the atmosphere of the Earth are in the form of the parent, 16O12C16O isotopologue, making it the most important isotopologue to study.
Carbon dioxide plays a crucial role in many areas of astronomical research as well, from the study of stars5,6 to the exploration of planetary atmospheres7,8,9: in our own solar system, carbon dioxide is the major constituent and thus determines the radiative balance of the atmospheres of the planets Mars and Venus. The detection and characterization of CO2 absorption features in exoplanetary spectra offer clues about the atmospheric composition, pressure, and temperature profiles of these distant worlds. CO2 was one of the first molecules detected in the atmosphere of an exoplanet10 and several recent observations have been made using the James Webb Space Telescope11. Within the dense molecular clouds that pervade the interstellar medium, CO2 serves as an important tracer of the physical and chemical conditions that govern the birth of new stars12,13. Emissions from the bending states of the CO2 isotopologues in the far infrared provide valuable information on the temperature, density, and kinematics of these star-forming regions12.
Remote sensing of the CO2 content of the Earth’s atmosphere is a major activity aimed at monitoring the carbon content of our atmosphere in increasing detail. Missions such as NASA’s OCO-2 and OCO-3 satellites and ESA’s planned CO2M satellite constellation have stringent requirements on laboratory spectroscopy results, required for the interpretation of their observations14,15. Similar accuracy is required for ground-based spectroscopic experiments such as TCCON (Total Carbon Column Observing Network)16.
CO2 spectra are important for medical17 and industrial applications18, as well. The study of CO2 spectra in plasma physics is widespread19, where there is particular emphasis on the use of plasma processes to valorize excess CO2 from the Earth’s atmosphere20.
Due to the importance of accurate high-resolution spectroscopic data related to carbon dioxide, they are available in several line-by-line spectroscopic databases, such as the Carbon Dioxide Spectroscopic Databank (CDSD-296)21, NASA Ames-202122, HITRAN202023, and ExoMol24. The present report on the spectroscopic data of 16O12C16O (626) is part of a long-term, ongoing project devoted to the construction of the most extensive empirical energy level datasets, calculated from measured line positions in high-resolution rovibrational spectra, for all isotopologues of carbon dioxide involving the 12C, 13C, 16O, 17O, and 18O isotopes. Empirical energy levels based on all the measured rovibrational transitions are already available for the carbon dioxide isotopologues 16O12C18O (628)25, 16O13C16O (636)26, 16O13C18O (638)27, 18O12C18O (828)28, 17O12C18O (728)28, and 18O13C18O (838)28 (see Table 1). In these projects, empirical energy levels are calculated using the MARVEL 4.0 (Measured Active Rotational-Vibrational Energy Levels) procedure29,30,31,32, built upon the theory of spectroscopic networks33,34. Statistical measures of these previous studies, mostly with respect to the Ames-202122 and CDSD-29621 datasets of energy levels are also given in Table 1. For the seven isotopologues studied thus far, agreement with the CDSD-296 data is significantly better, by more than an order of magnitude for the average absolute deviation.
The most important results of this study, obtained with the help of the MARVEL code for the 626 isotopologue of carbon dioxide, include the 626M24 dataset of validated experimental transitions and empirical rovibrational energy levels, and a large rovibrational line list, 626M24LL. All of these data will contribute not only to future spectroscopic measurements on carbon dioxide but also to the refinement of theoretical and computational spectroscopic models and the enhancement of spectroscopic line-by-line databases, such as HITRAN23 and ExoMol24,35.
Methods
Source data
References36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180 contain rovibrational transitions data considered during the MARVEL analysis of this study. The wavenumber range covered by these measurements is limited to 42.9 – 14 076 cm−1.
MARVEL
The MARVEL procedure29,30,31,32, used extensively during this study, starts with the careful collection, detailed examination, and subsequent validation of the positions of transitions in high-resolution (laboratory) spectra. The transitions collected are then used to construct a spectroscopic network (SN)33,34, whereby each energy level serves as a node and the nodes are interconnected by the observed transitions. The SN built allows the determination of empirical energy-level values along with educated estimates for their uncertainties32. Unlike the effective Hamiltonians widely used for spectroscopic analysis, the MARVEL approach is model-free. This has a number of advantages and, in particular, for the CO2 molecule with its many resonances, MARVEL does not require any special measures or extra parameters to characterize levels perturbed by “accidental” interactions with nearby states.
Ideally, the experimentally observed transitions allow the creation of a well-connected SN, linking all transitions to the ground state (defined as the state with no rovibrational excitation), called the root of the SN. However, because of the limited coverage offered by the experimental data, this is usually not the case. Therefore, in practice, the SN can become fragmented, resulting in a principal component, where all the nodes are linked to the root, and a number of isolated, so-called floating components with their own roots.
The MARVEL protocol allows for the detection of inconsistencies, that is, lines that are in conflict with the correct measurement data. This feature proves invaluable for identifying issues with experimental data that usually come from several sources, such as user errors made during data collection and analysis, incorrect assignments, or the use of different naming conventions.
Notation and quantum numbers
CO2 has three fundamental vibrational modes, conventionally denoted as ν1, ν2, and ν3, associated with the vibrational quantum numbers vi, i = 1, 2, and 3, respectively. The two-dimensional (degenerate) bending mode, ν2, is characterized by an angular momentum, described by the quantum number ℓ2. Herzberg’s notation is often used to assign energy levels in triatomics; in this notation, the vibrational states of CO2 are designated as \(({v}_{1}\,{v}_{2}^{{\ell }_{2}}\,{v}_{3})\). For the CO2 molecule with a linear equilibrium structure in its ground electronic state, there is a strong Fermi-resonance interaction between the states (\({v}_{1}\,{({v}_{2}+2)}^{{\ell }_{2}}\,{v}_{3}\)) and (\({v}_{1}+1\,{v}_{2}^{{\ell }_{2}}\,{v}_{3}\)). Therefore, it became customary to employ the so-called AFGL (Air Force Geophysics Laboratory) notation to denote the vibrational states and bands of CO2 isotopologues. In the AFGL notation181,182,183, the vibrational energy levels are designated by the quintuplet (v1 v2 ℓ2 v3 r), where r is the ranking index for states in Fermi resonance (the r index is used to distinguish the levels belonging to the same Fermi polyad). The lowest value of r, 1, is assigned to the energy level with the highest wavenumber (or frequency), and r increases for lower-energy levels. For example, the three vibrational states (2 00 0), (1 20 0), and (0 40 0) are in Fermi resonance with each other and have the AFGL vibrational descriptors (2 0 0 0 3), (2 0 0 0 2), and (2 0 0 0 1), respectively.
It is customary to use polyad numbers P to denote strongly interacting groups of vibrational states, decoupling them from the other vibrations. This is a useful concept, especially when effective Hamiltonians are formed. P is not a quantum number, but it behaves like one. For carbon dioxide, based on the approximate relations of the harmonic frequencies, ω1 ≈ 2ω2 and ω3 ≈ 3ω2, the widely accepted definition of P, also used in this study, is P = 2v1 + v2 + 3v3.
The quantum number J is used to denote the angular momentum associated with rotational and (when ℓ2 > 0) vibrational motion of the CO2 molecule. Transitions with ΔJ = − 1 and ΔJ = + 1 are called the P- and R-branch transitions, respectively, while the Q-branch transitions are associated with ΔJ = 0. P and R transitions occur in both the parallel and perpendicular bands, while the Q branch transitions only occur in the parallel bands, where the direction refers to the change in the dipole moment driving the transition relative to the linear equilibrium structure of the molecule. For the symmetric isotopologue 626, the Pauli principle means that symmetric vibrational states (those with even v3 values) only have even J levels, while anti-symmetric vibrational states (those with odd v3 values) have only odd J levels. Similarly, for states with even values of J + ℓ2 + v3 the rotationless parity is ‘e’, while for states with odd J + ℓ2 + v3 values the rotationless parity is ‘f’. The coupling of rotational and vibrational angular momentum means that J ≥ l2.
The upper and lower states involved in a transition are denoted as ′ and ″, respectively, and the P, R, and Q transitions are usually specified using the lower-state rotational quantum number (J″). For the purposes of the MARVEL analysis, each state is uniquely characterized using the set of seven descriptors (J v1 v2 ℓ2 v3 r e/f). This is the format followed by the data deposited in the Supplementary Material to this paper.
Data Records
The 626M24 dataset is available in an OSF (Open Science Framework) repository184. It contains (a) all experimentally measured transitions collected during this work, (b) all empirical rovibrational energy levels determined, and (c) an extensive line list derived from the levels. All validated transitions have positive wavenumber or frequency values, while transitions that had to be removed have negative values. The same repository contains a table describing the main characteristics of the 143 literature sources that contain the transitions collected and analyzed.
The file “626M24_segments.txt” is the segment input file utilized by the MARVEL code, where the unit of the line positions and their uncertainties are specified for each data source. The file “626M24_transitions.txt” contains the 44 828 input transitions, collected from Refs. 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180, used during the MARVEL analysis. In this file, each transition is characterized by (a) a line position (in units stored in the segment file), (b) an initial and an adjusted line-position uncertainty, (c) the rovibrational assignments for the upper and lower states (see the previous section for a description), and (d) a line tag, representing a unique identifier (each data source tag is based on the last two digits of the year of publication and the first two characters of the last names of the authors).
Of all the experimentally measured transitions only about half of them, 22 218, are unique. During the MARVEL analysis, 368 transitions had to be removed from our spectroscopic network; note, in particular, that all the measured transitions of 00TaPeTeLe107 had to be deleted. It is also worth mentioning that although the transitions of the sources 94Bailly93 and 97BaCaLa97 are included in the transition file, the transitions reported in them form floating components. Thus, we cannot independently validate them or determine the absolute values of the energy levels associated with them. Finally, it is important to add that although most of the transitions in the transition file “626M24_transitions.txt” are from measurements, the final dataset also contains calculated line positions. These sources are denoted by ‘_C’ in the tag. The reason to include these calculated line positions in the transitions list is that the uncertainty of these lines is several orders of magnitude smaller than that of other transitions measured in the given region and they help the analysis of the spectroscopic network of 626.
The empirical energy values, obtained for 8268 rovibrational states in the 0 − 20 654 cm−1 range, are placed in the file “626M24_energy_levels.txt”. Each energy level of this data file is characterized by (a) a rovibrational label, (b) an empirical (MARVEL) energy in hc cm−1, (c) an energy uncertainty in hc cm−1, and (d) the number of transitions incident to this state.
Using our empirical energies and the CDSD-29621, NASA Ames-202122, and HITRAN202023 line positions and intensities, an extended line list, named 626M24LL, was constructed, given in the file “626M24_line_list.txt”. Line intensities relate the probability of absorption by a given line at a specified temperature; here we adopt the standard HITRAN23 unit of cm molecule−1. This line list contains 285 503 dipole-allowed transitions in the range 147 − 19 909 cm−1, with room-temperature intensities down to 10−31 cm molecule−1. Columns (1) − (21) of the “626M24_line_list.txt” file contain the following information: (1) CDSD-296 line position, (2) AMES-21 line position, (3) HITRAN2020 line position, (4) MARVEL line position (generated from the MARVEL energy levels as Eup,MARVEL − Elow,MARVEL), (5) MARVEL uncertainty, (6) AMES-21 intensity (100% abundance assumed), (7) HITRAN2020 line intensity (scaled by natural abundance), (8-14) descriptors of the upper state, and (15-21) descriptors of the lower state. All line positions and uncertainties are in cm−1, the intensity values correspond to a temperature of 296 K. Beyond column (21) the line may contain a possible comment. Four types of comments are used: (a) ‘ONLY IN MARVEL’ means that this line can only be found in the experimental transitions dataset, but not in CDSD-296 and HITRAN2020. There are 290 such lines in the 626M24 dataset, typically with high v3 values (v3 > 6). (b) The 626M24 dataset contains 2506 lines that can be found ‘ONLY IN HITRAN’. Most of these lines (2134) are not assigned (their vibrational labels are –2 –2 –2 2 0). (c) When the deviation of the HITRAN2020 position from the CDSD-296 and/or MARVEL positions is larger than 0.01 cm−1, the comment ‘Incorrect HITRAN line position’ is used. These 565 HITRAN2020 lines should be reinvestigated and replaced with CDSD-296 or MARVEL positions. (d) When the deviation of the MARVEL position from the CDSD-296 position is larger than 0.005 cm−1, the comment ‘Conflict with MARVEL’ is used. There are 5110 such cases. They are divided into two groups. First, when both the lower and the upper energy levels are determined by at least three transitions, i.e., the MARVEL prediction is considered to be reliable, ‘!’ is used at the end of the comment. For example, three sources76,88,101 measured a line at 3181.915 cm−1, but the CDSD-296 position of this line is 3181.909 cm−1. Most of the cases (4427 occurrences) belong to the second group, where one of the MARVEL energy levels, typically the upper energy level, is defined by only one or two transitions. In this case, ‘?’ is placed at the end of the comment, denoting that it is possible that the experimentally measured line is not reliable. As a point of interest, note that while the initial dataset, “626M24_transitions.txt”, contains 816 transitions with uncertainties of less than 10−6 cm−1, the number of such transitions in the extended line list, “626M24_line_list.txt”, is 2101.
Finally, the file “626M24_MARVEL.exe” is a developer version of the MARVEL code, written in the C++ language. This version of the MARVEL code, distributed with the necessary input files ("626M24_transitions.txt” and “626M24_segments.txt”), was used to generate the numerical data of the 626M24 repository.
Technical Validation
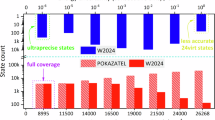
The principal validation of the 626M24 energy levels was performed via the MARVEL procedure (see Sec. 2.2). Basically, it involved an elaborate checking of the consistency of the experimentally measured transitions collected, in relation to their assignments, line positions, and uncertainties. Figure 1 shows the final experimental uncertainties of the validated rovibrational measurements of 16O12C16O as a function of the transition wavenumber. This figure shows that for 16O12C16O, one of the spectroscopically most studied molecules, (a) the uncertainties of the experimentally measured transitions cover almost eight orders of magnitude, from 1 × 10−9 to 10−1 cm−1, (b) the wavenumber range covered by the experiments is rather limited, only going up to 14 000 cm−1, and (c) there are no highly accurate measurements above 7000 cm−1.
The global MARVEL analysis resulted in the best rovibrational energy-level dataset, based on the presently available transitions. An important validation of the 626M24 energy values is their comparison with entries in standard databases. Comparison of the predicted line positions of 626M24 with those in the CDSD-29621, Ames-202122, and HITRAN202023 line catalogs is particularly important, as it allows additional validation of the energy dataset derived in this study. Furthermore, this comparison might reveal database entries that require further verification and/or modification.
Figure 2 shows the absolute deviations between the MARVEL data and those of CDSD-296 and Ames-2021. The MARVEL data show significantly better agreement with CDSD-296 (with a root-mean-square, rms, deviation of 0.0032 hc cm−1) than with Ames-2021 (with an rms value of 0.0238 hc cm−1, which is an order of magnitude higher). This is not surprising, as the CDSD-296 data are semi-empirical in nature. A comparison with the HITRAN2020 data can be found in the file “626M24_line_list.txt”.
Figure 3 shows the empirical rovibrational energy levels of the 16O12C16O molecule determined in this study as a function of the rotational quantum number J and the total energy; the vibrational structure can also be seen in the quadratic curves formed as a function of J. Figure 3 shows that the list of rotational energy levels for the ground vibrational state extends up to J = 108, but is incomplete, as the J = 94 and 96 states are not present in the MARVEL energy levels.
Table 2 lists the vibrational band origins of 16O12C16O determined in this study; as J = 0 levels only exist for vibrational states with ℓ2 = 0, these band origins are only for vibrational states which have ℓ2 = 0. It is perhaps surprising to see that there are only nine energies listed there and only four of them have an accuracy better than 1 × 10−3 hc cm−1.
Code availability
The developer version of the MARVEL code, used during the compilation and validation of the 626M24 dataset, is freely available as an OSF repository184.
References
Pearson, P. N. & Palmer, M. R. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406, 695–699, https://doi.org/10.1038/35021000 (2000).
Romps, D. M., Seeley, J. T. & Edman, J. P. Why the forcing from carbon dioxide scales as the logarithm of its concentration. J. Climate 35, 4027–4047, https://doi.org/10.1175/JCLI-D-21-0275.1 (2022).
Shine, K. P. & Perry, G. E. Radiative forcing due to carbon dioxide decomposed into its component vibrational bands. Q. J. R. Meteorol. 149, 1856–1866, https://doi.org/10.1002/qj.4485 (2023).
Calvin, K. et al. IPCC, 2023: Climate change 2023: Synthesis report. contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change [core writing team, H. Lee and J. Romero (eds.)]. IPCC, Geneva, Switzerland, Tech. rep. (2023).
Baylis-Aguirre, D. K., Creech-Eakman, M. J. & Güth, T. Mid-IR spectra of the M-type Mira variable R Tri observed with the Spitzer IRS. Mon. Not. Roy. Astron. Soc. 493, 807–814, https://doi.org/10.1093/mnras/staa322 (2020).
Fonfria, J. P., Montiel, E. J., Cernicharo, J., DeWitt, C. N. & Richter, M. J. Detection of infrared fluorescence of carbon dioxide in R Leonis with SOFIA/EXES. Astron. Astrophys. 643, L15, https://doi.org/10.1051/0004-6361/202039547 (2020).
Gilli, G. et al. Limb observations of CO2 and CO non-LTE emissions in the Venus atmosphere by VIRTIS/Venus Express. J. Geophys. Res.-Planets 114, E00B29, https://doi.org/10.1029/2008JE003112 (2009).
Snels, M., Stefani, S., Grassi, D., Piccioni, G. & Adriani, A. Carbon dioxide opacity of the Venus’ atmosphere. Planet Space Sci. 103, 347–354, https://doi.org/10.1016/j.pss.2014.08.002 (2014).
Trokhimovskiy, A. et al. First observation of the magnetic dipole CO2 main isotopologue absorption band at 3.3 μm in the atmosphere of Mars by the ExoMars Trace Gas Orbiter ACS instrument. Astron. Astrophys. 639, A142, https://doi.org/10.1051/0004-6361/202038134 (2020).
Swain, M. R. et al. Molecular signatures in the near-infrared dayside spectrum of HD 189733b. Astrophys. J. Lett. 690, L114–L117, https://doi.org/10.1088/0004-637X/690/2/L114 (2009).
Ahrer, E.-M. et al. Identification of carbon dioxide in an exoplanet atmosphere. Nature 614, 649–652, https://doi.org/10.1038/s41586-022-05269-w (2023).
Boonman, A. M. S. et al. Gas-phase \({{\rm{CO}}}_{{\mathsf{2}}}\), \({{\rm{C}}}_{{\mathsf{2}}}{{\rm{H}}}_{{\mathsf{2}}}\), and HCN toward Orion-KL*. Astron. Astrophys. 399, 1047–1061, https://doi.org/10.1051/0004-6361:20021799 (2003).
Boonman, A. M. S., van Dishoeck, E. F., Lahuis, F. & Doty, S. D. Gas-phase CO2 toward massive protostars. Astron. Astrophys. 399, 1063–1072, https://doi.org/10.1051/0004-6361:20021868 (2003).
Hobbs, J. M. et al. Spectroscopic uncertainty impacts on OCO-2/3 retrievals of XCO2. J. Quant. Spectrosc. Radiat. Transf. 257, 107360, https://doi.org/10.1016/j.jqsrt.2020.107360 (2020).
Noel, S. et al. Greenhouse gas retrievals for the CO2M mission using the FOCAL method: first performance estimates. Atmos. Meas. Tech. 17, 2317–2334, https://doi.org/10.5194/amt-17-2317-2024 (2024).
Laughner, J. L. et al. The Total Carbon Column Observing Network’s GGG2020 data version. Earth Sys. Sci. Data 16, 2197–2260, https://doi.org/10.5194/essd-16-2197-2024 (2024).
Ageev, V. G. & Nikiforova, O. Y. Optoacoustic determination of carbon dioxide concentration in exhaled breath in various human diseases. J. Appl. Spectrosc. 83, 820–825, https://doi.org/10.1007/s10812-016-0369-z (2016).
Evseev, V., Fateev, A. & Clausen, S. High-resolution transmission measurements of CO2 at high temperatures for industrial applications. J. Quant. Spectrosc. Radiat. Transf. 113, 2222–2233, https://doi.org/10.1016/j.jqsrt.2012.07.015 (2012).
Du, Y., Tsankov, T. V., Luggenhöelscher, D. & Czarnetzki, U. Time evolution of CO2 ro-vibrational excitation in a nanosecond discharge measured with laser absorption spectroscopy. J. Phys.D-Appl. Phys. 54, 365201, https://doi.org/10.1088/1361-6463/ac03e7 (2021).
Snoeckx, R. & Bogaerts, A. Plasma technology - a novel solution for CO2 conversion? Chem. Soc. Rev. 46, 5805–5863, https://doi.org/10.1039/c6cs00066e (2017).
Tashkun, S. A., Perevalov, V. I., Gamache, R. R. & Lamouroux, J. CDSD-296, high-resolution carbon dioxide spectroscopic databank: An update. J. Quant. Spectrosc. Radiat. Transf. 228, 124–131, https://doi.org/10.1016/j.jqsrt.2019.03.001 (2019).
Huang, X., Schwenke, D. W., Freedman, R. S. & Lee, T. J. Ames-2021 CO2 dipole moment surface and IR line lists: Toward 0.1% uncertainty for CO2 IR intensities. J. Phys. Chem. A 126, 5940–5964, https://doi.org/10.1021/acs.jpca.2c01291 (2022).
Gordon, I. E. et al. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 277, 107949, https://doi.org/10.1016/j.jqsrt.2021.107949 (2022).
Yurchenko, S. N., Mellor, T. M., Freedman, R. S. & Tennyson, J. ExoMol molecular line lists XXXIX: Ro-vibrational molecular line list for CO2. Mon. Not. Roy. Astron. Soc. 496, 5282–5291, https://doi.org/10.1093/mnras/staa1874 (2020).
Alatoom, D. et al. MARVEL analysis of high-resolution rovibrational spectra of 16O12C18O. J. Comput. Chem. 45, 2558-2573, https://doi.org/10.1002/jcc.27453 (2024).
Ibrahim, M. T. I. et al. MARVEL analysis of high-resolution rovibrational spectra of 16O13C16O. J. Comput. Chem. 45, 969–984, https://doi.org/10.1002/jcc.27266 (2024).
Azzam, A. A. A., Tennyson, J., Yurchenko, S. N., Furtenbacher, T. & Császár, A. G. MARVEL analysis of high-resolution rovibrational spectra of 16O13C18O, J. Comput. Chem. 46, e27541, https://doi.org/10.1002/jcc.27541 (2025).
Azzam, A. A. A. et al. MARVEL analysis of high-resolution rovibrational spectra of the 18O12C18O, 17O12C18O, and 18O13C18O isotopologues of carbon dioxide. J. Mol. Spectrosc. 405, 111947, https://doi.org/10.1016/j.jms.2024.111947 (2024).
Császár, A. G., Czakó, G., Furtenbacher, T. & Mátyus, E. An active database approach to complete rotational–vibrational spectra of small molecules. Annu. Rep. Comput. Chem. 3, 155–176, https://doi.org/10.1016/S1574-1400(07)03009-5 (2007).
Furtenbacher, T., Császár, A. G. & Tennyson, J. MARVEL: measured active rotational-vibrational energy levels. J. Mol. Spectrosc. 245, 115–125, https://doi.org/10.1016/j.jms.2007.07.005 (2007).
Furtenbacher, T. & Császár, A. G. MARVEL: measured active rotational-vibrational energy levels. II. Algorithmic improvements. J. Quant. Spectrosc. Radiat. Transf. 113, 929–935, https://doi.org/10.1016/j.jqsrt.2012.01.005 (2012).
Tennyson, J., Furtenbacher, T., Yurchenko, S. N. & Császár, A. G. Empirical rovibrational energy levels for nitrous oxide. J. Quant. Spectrosc. Radiat. Transf. 316, 108902, https://doi.org/10.1016/j.jqsrt.2024.108902 (2024).
Császár, A. G. & Furtenbacher, T. Spectroscopic networks. J. Mol. Spectrosc. 266, 99 – 103, https://doi.org/10.1016/j.jms.2011.03.031 (2011).
Furtenbacher, T. & Császár, A. G. The role of intensities in determining characteristics of spectroscopic networks. J. Mol. Struct. 1009, 123–129, https://doi.org/10.1016/j.molstruc.2011.10.057 (2012).
Tennyson, J. et al. The 2024 release of the ExoMol database: molecular line lists for exoplanet and other hot atmospheres. J. Quant. Spectrosc. Radiat. Transf. 326, 109083, https://doi.org/10.1016/j.jqsrt.2024.109083 (2024).
Martin, P. E. & Barker, E. F. The infrared absorption spectrum of carbon dioxide. Phys. Rev. 41, 291–303, https://doi.org/10.1103/PhysRev.41.291 (1932).
Nielsen, A. H. & Yao, Y. T. The analysis of the vibration-rotation band ω3 for \({}^{12}{\rm{C}}{}^{16}{\rm{O}}_{2}\) and \({}^{13}{\rm{C}}{}^{16}{\rm{O}}_{2}\). Phys. Rev. 68, 173–180, https://doi.org/10.1103/PhysRev.68.173 (1945).
Goldberg, L., Mohler, O. C., Mcmath, R. R. & Pierce, A. K. Carbon dioxide in the infra-red solar spectrum. Phys. Rev. 76, 1848–1858, https://doi.org/10.1103/PhysRev.76.1848 (1949).
Herzberg, G. & Herzberg, L. Rotation-vibration spectra of diatomic and simple polyatomic molecules with long absorbing paths XI. The spectrum of carbon dioxide (CO2) below 1.25 μm. J. Opt. Soc. Am. 43, 1037–1044, https://doi.org/10.1364/JOSA.43.001037 (1953).
Rossmann, K., Rao, K. N. & Nielsen, H. H. Infrared spectrum and molecular constants of carbon dioxide. Part I. ν2 of 12C16O2 at 15 μ. J. Chem. Phys. 24, 103–105, https://doi.org/10.1063/1.1700807 (1956).
Madden, R. P. A high-resolution study of CO2 absorption spectra between 15 and 18 microns. J. Chem. Phys. 35, 2083–2097, https://doi.org/10.1063/1.1732212 (1961).
Plyler, E. K., Tidwell, E. D. & Benedict, W. S. Absorption bands of carbon dioxide from 2.8–4.2 μ. J. Opt. Soc. Am. 52, 1017–1022, https://doi.org/10.1364/JOSA.52.001017 (1962).
Gordon, H. R. & McCubbin, T. K. The 15-micron bands of 12C16O2. J. Mol. Spectrosc. 18, 73–82, https://doi.org/10.1016/0022-2852(65)90063-9 (1965).
Gordon, H. R. The infrared spectrum of CO2 in the 2.8 and 15 micron regions, Ph.D. thesis, The Pennsylvania State University https://www.proquest.com/openview/59dcaa567b3cb6b908786d3699a70cc9/1?pq-origsite=gscholar&cbl=18750&diss=y (1965).
Gordon, H. R. & McCubbin, T. K. The 2.8-micron bands of CO2. J. Mol. Spectrosc. 19, 137–154, https://doi.org/10.1016/0022-2852(66)90237-2 (1966).
Hartmann, B. & Kleman, B. Laser lines from CO2 in the 11-18 micron region. Can. J. Phys. 44, 1609–1612, https://doi.org/10.1139/p66-134 (1966).
Hahn, Y. H. The absorption and emission spectra of carbon-dioxide at 4.3 microns, Ph.D. thesis, The Pennsylvania State University https://www.proquest.com/openview/074bf2696a7b47d2793ca1d620a02193/1?pq-origsite=gscholar&cbl=18750&diss=y (1967).
Oberly, R., Rao, K. N., Hahn, Y. H. & McCubbin, T. K. Bands of carbon dioxide in the region of 4.3 microns. J. Mol. Spectrosc. 25, 138–165, https://doi.org/10.1016/0022-2852(68)80002-5 (1968).
Gray Young, L. D., Young, A. T. & Schorn, R. A. Improved constants for the 7820 Å and 7883 Å bands of CO2. J. Quant. Spectrosc. Radiat. Transfer 10, 1291–1300, https://doi.org/10.1016/0022-4073(70)90011-7 (1970).
Blaney, T. G. et al. Absolute frequency measurement of the R(12) Transition of CO2 at 9.3 μm. Nature 244, 504–504, https://doi.org/10.1038/244504a0 (1973).
Evenson, K. M., Wells, J. S., Petersen, F. R., Danielson, B. L. & Day, G. W. Accurate frequencies of molecular transitions used in laser stabilization: the 3.39-μm transition in CH4 and the 9.33- and 10.18-μm transitions in CO2. Appl. Phys. Lett. 22, 192–195, https://doi.org/10.1063/1.1654607 (1973).
Schiffner, G. Improved determination of accurate CO2 laser transition frequencies and their standard deviation. Opto-Electronics 5, 411–413, https://doi.org/10.1007/bf01418076 (1973).
McCubbin, T. K., Pliva, J., Pulfrey, R., Telfair, W. & Todd, T. The emission spectrum of 12C16O2 from 4.2 to 4.7 microns. J. Mol. Spectrosc. 49, 136–156, https://doi.org/10.1016/0022-2852(74)90103-9 (1974).
Toth, R. A. Wavenumbers, strengths, and self-broadened widths of CO2 at 3 μm. J. Mol. Spectrosc. 53, 1–14, https://doi.org/10.1016/0022-2852(74)90256-2 (1974).
Dupre-Maquaire, J. & Pinson, P. Emission spectrum of CO2 in the 9.6 μm region. J. Mol. Spectrosc. 62, 181–191, https://doi.org/10.1016/0022-2852(76)90348-9 (1976).
Reid, J. & Siemsen, K. New CO2 laser bands in the 9-11-μm wavelength region. Appl. Phys. Lett. 29, 250–251, https://doi.org/10.1063/1.89033 (1976).
Monchalin, J. P., Kelly, M. J., Thomas, J. E., Kurnit, N. A. & Javan, A. Accurate wavelength measurement of P-branch transitions of the \(0{1}^{1}1-{[1{1}^{1}0,0{3}^{1}0]}_{{\rm{I}}}\) band of 12C16O2 and determination of the band parameters. J. Mol. Spectrosc. 64, 491–494, https://doi.org/10.1016/0022-2852(77)90233-8 (1977).
Nereson, N. G. & Flicker, H. Wavenumber measurement of weak CO2 laser lines around 10.6 μm. Opt. Commun. 23, 171–176, https://doi.org/10.1016/0030-4018(77)90299-1 (1977).
Whitford, B. G., Siemsen, K. J. & Reid, J. Heterodyne frequency measurements of CO2 laser hot-band transitions. Opt. Commun. 22, 261–264, https://doi.org/10.1016/S0030-4018(97)90004-3 (1977).
Siemens, K. J. & Whitford, B. G. Heterodyne frequency measurements of CO2 laser sequence-band transitions. Opt. Commun. 22, 11–16, https://doi.org/10.1016/0030-4018(77)90235-8 (1977).
Arcas, P. & Arié, E. Absorption spectrum of CO2 in the 4.82-μm region. J. Mol. Spectrosc. 70, 134–142, https://doi.org/10.1016/0022-2852(78)90015-2 (1978).
Baldacci, A., Devi, V. M., Chen, D.-W., Rao, K. N. & Fridovich, B. Absorption spectrum of carbon dioxide at 4.3 μm. J. Mol. Spectrosc. 70, 143–159, https://doi.org/10.1016/0022-2852(78)90016-4 (1978).
Roney, P. L., Findlay, F. D., Buijs, H. L., Cann, M. W. P. & Nicholls, R. W. Carbon dioxide spectral line frequencies for the 43-μm region. Appl. Opt. 17, 2599, https://doi.org/10.1364/AO.17.002599 (1978).
Freed, C., Bradley, L. & O’Donnell, R. Absolute frequencies of lasing transitions in seven CO2 isotopic species. IEEE J. Quantum Electron. 16, 1195–1206, https://doi.org/10.1109/jqe.1980.1070392 (1980).
Guelachvili, G. High-resolution Fourier spectra of carbon dioxide and three of its isotopic species near 4.3 μm. J. Mol. Spectrosc. 79, 72–83, https://doi.org/10.1016/0022-2852(80)90293-3 (1980).
Maillard, J. P., Cuisenier, M., Arcas, P., Arié, E. & Amiot, C. Infrared spectrum and molecular constants of CO2 in the 1.4-1.7 μm atmospheric window by very high resolution Fourier transform spectroscopy. Can. J. Phys. 58, 1560–1569, https://doi.org/10.1139/p80-205 (1980).
Paso, R., Kauppinen, J. & Anttila, R. Infrared spectrum of CO2 in the region of the bending fundamental ν2. J. Mol. Spectrosc. 79, 236–253, https://doi.org/10.1016/0022-2852(80)90304-5 (1980).
Pine, A. S. & Guelachvili, G. R-branch head of the ν3 band of CO2 at elevated temperatures. J. Mol. Spectrosc. 79, 84–89, https://doi.org/10.1016/0022-2852(80)90294-5 (1980).
Bailly, D., Farrenq, R., Guelachvili, G. & Rossetti, C. 12C16O2 analysis of emission Fourier spectra in the 4.5-μm region: Rovibrational transitions \(0{v}_{2}^{\ell }{v}_{3}-0{v}_{2}^{\ell }({v}_{3}-1),{v}_{2}=\ell \). J. Mol. Spectrosc. 90, 74–105, https://doi.org/10.1016/0022-2852(81)90334-9 (1981).
Kauppinen, J., Jolma, K. & Horneman, V. M. New wave-number calibration tables for H2O, CO2, and OCS lines between 500 and 900 cm−1. Appl Opt. 21, 3332–6, https://doi.org/10.1364/AO.21.003332 (1982).
Arcas, P., Arie, E., Cuisenier, M. & Maillard, J. P. The infrared spectrum and molecular constants of CO2 in the 2 μm region. Can. J. Phys. 61, 857–866, https://doi.org/10.1139/p83-105 (1983).
Jolma, K., Kauppinen, J. & Horneman, V. M. Vibration-rotation bands of CO2 and OCS in the region 540-890 cm−1. J. Mol. Spectrosc. 101, 300–305, https://doi.org/10.1016/0022-2852(83)90135-2 (1983).
Petersen, F. R., Beaty, E. C. & Pollock, C. R. Improved rovibrational constants and frequency tables for the normal laser bands of 12C16O2. J. Mol. Spectrosc. 102, 112–122, https://doi.org/10.1016/0022-2852(83)90231-X (1983).
Devi, V. M., Rinsland, C. P. & Benner, D. C. Absolute intensity measurements of CO2 bands in the 2395-2680-cm−1 region. Appl. Opt. 23, 4067, https://doi.org/10.1364/AO.23.004067 (1984).
Rinsland, C. P. & Benner, D. C. Absolute intensities of spectral lines in carbon dioxide bands near 2050 cm−1. Appl. Opt. 23, 4523–4528, https://doi.org/10.1364/AO.23.004523 (1984).
Rinsland, C. P. et al. Atlas of high resolution infrared spectra of carbon dioxide. Appl. Opt. 23, 2051–2052, https://doi.org/10.1364/AO.23.002051 (1984).
Petersen, F. R., Wells, J. S., Siemsen, K. J., Robinson, A. M. & Maki, A. G. Heterodyne frequency measurements and analysis of CO2 laser hot band transitions. J. Mol. Spectrosc. 105, 324–330, https://doi.org/10.1016/0022-2852(84)90222-4 (1984).
Benner, D. C. & Rinsland, C. P. Identification and intensities of the “forbidden” 3\({\nu }_{2}^{3}\) band of 12C16O2. J. Mol. Spectrosc. 112, 18–25, https://doi.org/10.1016/0022-2852(85)90187-0 (1985).
Brown, L. R. & Toth, R. A. Comparison of the frequencies of NH3, CO2, H2O, N2O, CO, and CH4 as infrared calibration standards. J. Opt. Soc. Am. B 2, 842–856, https://doi.org/10.1364/JOSAB.2.000842 (1985).
Rinsland, C. P., Benner, D. C. & Devi, V. M. Measurements of absolute line intensities in carbon dioxide bands near 5.2 μm. Appl. Opt. 24, 1644–1650, https://doi.org/10.1364/AO.24.001644 (1985).
Bradley, L., Soohoo, K. & Freed, C. Absolute frequencies of lasing transitions in nine CO2 isotopic species. IEEE J. Quantum Electron. 22, 234–267, https://doi.org/10.1109/JQE.1986.1072967 (1986).
Chardonnet, C., van Lerberghe, A. & Bordé, C. J. Absolute frequency determination of super-narrow CO2 saturation peaks observed in an external absorption cell. Opt. Commun. 58, 333–337, https://doi.org/10.1016/0030-4018(86)90239-7 (1986).
Esplin, M. P. & Rothman, L. S. Spectral measurements of high-temperature isotopic carbon dioxide in the 4.5- and 2.8 μm regions. J. Mol. Spectrosc. 116, 351–363, https://doi.org/10.1016/0022-2852(86)90132-3 (1986).
Esplin, M. P. et al. Carbon dioxide line positions in the 2.8 and 4.3 micron regions at 800 Kelvin, Tech. Rep. AFGL-TR-86-0046, Utah State University https://apps.dtic.mil/sti/citations/ADA173808 (1986).
Guelachvili, G., Rao, K. R. Handbook of Infrared Standards, Academic Press, (1986).
Rinsland, C. P., Benner, D. C. & Devi, V. M. Absolute line intensities in CO2 bands near 4.8 μm. Appl. Opt. 25, 1204–1214, https://doi.org/10.1364/AO.25.001204 (1986).
Blanquet, G., Walrand, J. & Teffo, J. L. Frequency diode laser measurement of a very weak Q branch of CO2 near 864 cm−1. Appl. Opt. 27, 2098–2099, https://doi.org/10.1364/ao.27.002098 (1988).
Benner, D. C., Devi, V. M., Rinsland, C. P. & Ferry-Leeper, P. S. Absolute intensities of CO2 lines in the 3140-3410-cm−1 spectral region. Appl. Opt. 27, 1588–1597, https://doi.org/10.1364/AO.27.001588 (1988).
Ouazzany, Y., Boquillon, J. P. & Schrötter, H. W. High resolution CARS spectrum and analysis of the ν1 band Q-branch of carbon dioxide. Mol. Phys. 63, 769–777, https://doi.org/10.1080/00268978800100551 (1988).
Hamdouni, A. & Dana, V. Absolute line intensities in the 20002 ← 11102 and 12201 ← 03301 bands of 12C16O2. Appl. Opt. 29, 1570–1572, https://doi.org/10.1364/AO.29.001570 (1990).
Giver, L. P. & Chackerian, C. Rovibrational intensities for the \({(3{1}^{1}0)}_{{\rm{IV}}}\leftarrow (0{0}^{0}0)\) band of 12C16O2 at 4416 cm−1. J. Mol. Spectrosc. 148, 80–85, https://doi.org/10.1016/0022-2852(91)90036-a (1991).
Groh, A., Goddon, D., Schneider, M., Zimmermann, W. & Urban, W. Sub-doppler heterodyne frequency measurements on the CO2 10011-00001 vibrational band: New reference lines near 3714 cm−1. J. Mol. Spectrosc. 146, 161–168, https://doi.org/10.1016/0022-2852(91)90379-O (1991).
Bailly, D. 12C16O2 in emission in the 4.5-μm region: Some more rovibrational transitions \({v}_{1}{v}_{2}^{{\prime} }{v}_{3}\leftarrow {v}_{1}{v}_{2}^{{\prime} }({v}_{3}-1)\) occurring between very few populated levels. J. Mol. Spectrosc. 166, 1–11, https://doi.org/10.1006/jmsp.1994.1166 (1994).
Maki, A. G., Chou, C. C., Evenson, K. M., Zink, L. R. & Shy, J. T. Improved molecular constants and frequencies for the CO2 laser from new high-J regular and hot-band frequency measurements. J. Mol. Spectrosc. 167, 211–224, https://doi.org/10.1006/jmsp.1994.1227 (1994).
Chou, C. C. et al. Frequency measurements and molecular constants of CO2 0002-[1001,0201]I,II sequence band transitions. J. Mol. Spectrosc. 172, 233–242, https://doi.org/10.1006/jmsp.1995.1171 (1995).
Steyert, D. W., Weber, M., Sirota, J. M. & Reuter, D. C. Absolute intensities for the Q-branch of the \(4{v}_{2}^{2}\leftarrow 3{v}_{2}^{3}\) (581.776 cm−1) band in carbon dioxide. J. Quant. Spectrosc. Rad. Transf. 54, 815–818, https://doi.org/10.1016/0022-4073(95)00101-p (1995).
Bailly, D., Camy-Peyret, C. & Lanquetin, R. Temperature measurement in flames through CO2 and CO emission: New highly excited levels of CO2. J. Mol. Spectrosc. 182, 10–17, https://doi.org/10.1006/jmsp.1996.7205 (1997).
Bernard, V., Nogues, G., Daussy, C., Constantin, L. & Chardonnet, C. CO2 laser stabilized on narrow saturated absorption resonances of CO2, improved absolute frequency measurements. Metrologia 34, 313, https://doi.org/10.1088/0026-1394/34/4/4 (1997).
Bailly, D. 12C16O2 in emission in the 4.5-μm region: Transitions \({v}_{1}{v}_{2}^{\ell }{v}_{3}\leftarrow {v}_{1}{v}_{2}^{\ell }({v}_{3}-1)\) with (2v1 + v2) = 6 occurring between highly excited vibrational states. J. Mol. Spectrosc. 192, 257–262, https://doi.org/10.1006/jmsp.1998.7667 (1998).
Bailly, D., Tashkun, S. A., Perevalov, V. I., Teffo, J. L. & Arcas, P. H. CO2 emission in the 4-μm region: The \({(2{1}^{1}1)}_{3}\leftarrow {(2{1}^{1}0)}_{3}\) transition revisited. J. Mol. Spectrosc. 190, 1–6, https://doi.org/10.1006/jmsp.1998.7534 (1998).
Devi, V. M., Benner, D. C., Rinsland, C. P. & Smith, M. A. H. Absolute rovibrational intensities of 12C16O2 absorption bands in the 3090-3850 cm−1 spectral region. J. Quant. Spectrosc. Rad. Transf. 60, 741–770, https://doi.org/10.1016/s0022-4073(98)00080-6 (1998).
Frech, B. et al. Frequency measurements of saturated-fluorescence-stabilized CO2 laser lines: comparison with an OsO4-stabilized CO2 laser standard. Appl. Phys. B 67, 217–221, https://doi.org/10.1007/s003400050496 (1998).
Bailly, D., Tashkun, S., Perevalov, V. I., Teffo, J. L. & Arcas, P. Flame spectra of CO2 in the 3-μm region. J. Mol. Spectrosc. 197, 114–119, https://doi.org/10.1006/jmsp.1999.7883 (1999).
Campargue, A., Bailly, D., Teffo, J. L., Tashkun, S. A. & Perevalov, V. I. The ν1 + 5ν3 dyad of 12CO2 and 13CO2. J. Mol. Spectrosc. 193, 204–212, https://doi.org/10.1006/jmsp.1998.7718 (1999).
Acef, O., Michaud, F. & Rovera, G. V. Accurate determination of OsO4 absolute frequency grid at 28/29 THz. IEEE Trans. Instr. Meas. 48, 567–570 (1999).
Predoi-Cross, A., Luo, C., Berman, R., Drummond, J. R. & May, A. D. Line strengths, self-broadening, and line mixing in the 200 ← 0110 (Σ ← Π) Q branch of carbon dioxide. J. Chem. Phys. 112, 8367–8377, https://doi.org/10.1063/1.481480 (2000).
Tashkun, S. A. et al. 13C16O2: Global treatment of vibrational-rotational spectra and first observation of the 2ν1 + 5ν3 and ν1 + 2ν2 + 5ν3 absorption bands. J. Mol. Spectrosc. 200, 162–176, https://doi.org/10.1006/jmsp.2000.8057 (2000).
Teffo, J.-L. et al. Line intensities of 12C16O2 in the 1.2-1.4 μm spectral region. J. Mol. Spectrosc. 201, 249–255, https://doi.org/10.1006/jmsp.2000.8092 (2000).
Chou, C.-C., Lin, T. & Shy, J.-T. Wavenumber measurements of CO2 transitions in 1.5-μm atmospheric window using an external-cavity diode laser. J. Mol. Spectrosc. 205, 122–127, https://doi.org/10.1006/jmsp.2000.8242 (2001).
Weirauch, G. & Campargue, A. Spectroscopy and intensity measurements of the 3ν1+3ν3 tetrad of 12CO2 and 13CO2. J. Mol. Spectrosc. 207, 263–268, https://doi.org/10.1006/jmsp.2001.8342 (2001).
Vander Auwera, J., El Hachtouki, R. & Brown, L. R. Absolute line wavenumbers in the near infrared: 12C2H2 and 12C16O2. Mol. Phys. 100, 3563–3576, https://doi.org/10.1080/00268970210162880 (2002).
Ding, Y., Bertseva, E. & Campargue, A. Note: The 2ν1 + 3ν3 triad of 12CO2. J. Mol. Spectrosc. 212, 219–222, https://doi.org/10.1006/jmsp.2002.8553 (2002).
Devi, V. M., Benner, D. C., Smith, M. A. H. & Rinsland, C. P. Nitrogen broadening and shift coeffcients in the 4.2-4.5 micron bands of CO2. J. Quant. Spectrosc. Rad. Transf. 76, 289–307 (2003).
Giver, L. P., Brown, L. R., Chackerian, C. & Freedman, R. S. The rovibrational intensities of five absorption bands of between 5218 and 5349 cm−1. J. Quant. Spectrosc. Rad. Transf. 78, 417–436, https://doi.org/10.1016/s0022-4073(02)00277-7 (2003).
Miller, C. E. & Brown, L. R. Near infrared spectroscopy of carbon dioxide I. 16O12C16O line positions. J. Mol. Spectrosc. 228, 329–354, https://doi.org/10.1016/j.jms.2003.11.001 (2004).
André, F., Perrin, M. Y. & Taine, J. FTIR measurements of 12C16O2 line positions and intensities at high temperature in the 3700-3750 cm−1 spectral region. J. Mol. Spectrosc. 228, 187–205, https://doi.org/10.1016/j.jms.2004.07.004 (2004).
Amy-Klein, A., Vigué, H. & Chardonnet, C. Absolute frequency measurement of 12C16O2 laser lines with a femtosecond laser comb and new determination of the 12C16O2 molecular constants and frequency grid. J. Mol. Spectrosc. 228, 206–212, https://doi.org/10.1016/j.jms.2004.07.005 (2004).
Ding, Y., Campargue, A., Bertseva, E., Tashkun, S. & Perevalov, V. I. Highly sensitive absorption spectroscopy of carbon dioxide by ICLAS-VeCSEL between 8800 and 9530 cm−1. J. Mol. Spectrosc. 231, 117–123, https://doi.org/10.1016/j.jms.2004.12.008 (2005).
Garnache, A., Liu, A., Cerutti, L. & Campargue, A. Intracavity laser absorption spectroscopy with a vertical external cavity surface emitting laser at 2.3 μm: Application to water and carbon dioxide. Chem. Phys. Lett. 416, 22–27, https://doi.org/10.1016/j.cplett.2005.09.028 (2005).
Lucchesini, A. & Gozzini, S. Diode laser overtone spectroscopy of CO2 at 780 nm. J. Quant. Spectrosc. Radiat. Transf. 96, 289–299, https://doi.org/10.1016/j.jqsrt.2005.03.005 (2005).
Mazzotti, D., Cancio, P., Giusfredi, G., De Natale, P. & Prevedelli, M. Frequency-comb-based absolute frequency measurements in the mid-infrared with a difference-frequency spectrometer. Opt. Lett. 30, 997, https://doi.org/10.1364/ol.30.000997 (2005).
Majcherova, Z. et al. High-sensitivity CW-cavity ringdown spectroscopy of 12CO2 near 1.5 μm. J. Mol. Spectrosc. 230, 1–21, https://doi.org/10.1016/j.jms.2004.09.011 (2005).
Shao, J. et al. Highly sensitive diode laser absorption measurements of CO2 near 1.57 μm at room temperature. Opt. Appl. XXXV, 49–57 (2005).
Wang, L., Perevalov, V. I., Tashkun, S. A., Liu, A. W. & Hu, S. M. Absorption spectra of 12C16O2 and 13C16O2 near 1.05 μm. J. Mol. Spectrosc. 233, 297–300, https://doi.org/10.1016/j.jms.2005.07.008 (2005).
Perevalov, B. V. et al. CW-cavity ringdown spectroscopy of carbon dioxide isotopologues near 1.5 μm. J. Mol. Spectrosc. 238, 241–255, https://doi.org/10.1016/j.jms.2006.05.009 (2006).
Toth, R. A., Brown, L. R., Miller, C. E., Devi, V. M. & Benner, D. C. Line strengths of 12C16O2 : 4550-7000 cm−1. J. Mol. Spectrosc. 239, 221–242, https://doi.org/10.1016/j.jms.2006.08.001 (2006).
Horneman, V.-M. High accurate peak positions for calibration purposes with the lowest fundamental bands ν2 of N2O and CO2. J. Mol. Spectrosc. 241, 45–50, https://doi.org/10.1016/j.jms.2006.10.014 (2007).
Lucchesini, A. & Gozzini, S. Diode laser spectroscopy of CO2 at 790 nm. J. Quant. Spectrosc. Rad. Transf. 103, 74–82, https://doi.org/10.1016/j.jqsrt.2006.06.009 (2007).
Borri, S. et al. Lamb-dip-locked quantum cascade laser for comb-referenced IR absolute frequency measurements. Opt. Expr. 16, 11637, https://doi.org/10.1364/oe.16.011637 (2008).
Perevalov, B. V., Kassi, S., Perevalov, V. I., Tashkun, S. A. & Campargue, A. High sensitivity CW-CRDS spectroscopy of 12C16O2, 16O12C17O and 16O12C18O between 5851 and 7045 cm−1: Line positions analysis and critical review of the current databases. J. Mol. Spectrosc. 252, 143–159, https://doi.org/10.1016/j.jms.2008.06.012 (2008).
Kassi, S., Song, K. F. & Campargue, A. High sensitivity CW-cavity ring down spectroscopy of 12CO2 near 1.35 μm (I): line positions. J. Quant. Spectrosc. Radiat. Transf. 110, 1801–1814, https://doi.org/10.1016/j.jqsrt.2009.04.010 (2009).
Campargue, A., Song, K. F., Mouton, N., Perevalov, V. I. & Kassi, S. High sensitivity CW-Cavity Ring Down Spectroscopy of five 13CO2 isotopologues of carbon dioxide in the 1.26-1.44 μm region (I): Line positions. J. Quant. Spectrosc. Radiat. Transf. 111, 659–674, https://doi.org/10.1016/j.jqsrt.2009.11.013 (2010).
Pastor, P. C., Galli, I., Giusfredi, G., Mazzotti, D., De Natale, P. Saturated-absorption cavity ring-down spectroscopy, in: Frontiers in Optics 2010/Laser Science XXVI, Vol. 104 of FiO, OSA, 2010, p. FTuL4. https://doi.org/10.1364/fio.2010.ftul4
Song, K. F., Kassi, S., Tashkun, S. A., Perevalov, V. I. & Campargue, A. High sensitivity CW-cavity ring down spectroscopy of 12CO2 near 1.35 μm (II): New observations and line intensities modeling. J. Quant. Spectrosc. Radiat. Transf. 111, 332–344, https://doi.org/10.1016/j.jqsrt.2009.09.004 (2010).
Gatti, D. et al. Absolute frequency spectroscopy of CO2 lines at around 2.09 μm by combined use of an Er:fiber comb and a Ho:YLF amplifier. Opt. Lett. 36, 3921–3, https://doi.org/10.1364/OL.36.003921 (2011).
Gatti, D. et al. High-precision molecular interrogation by direct referencing of a quantum-cascade-laser to a near-infrared frequency comb. Opt. Expr. 19, 17520, https://doi.org/10.1364/oe.19.017520 (2011).
Song, K.-F. et al. High sensitivity cavity ring down spectroscopy of CO2 overtone bands near 790 nm. J. Quant. Spectrosc. Rad. Transf. 112, 761–768, https://doi.org/10.1016/j.jqsrt.2010.11.006 (2011).
Cappelli, F. et al. Subkilohertz linewidth room-temperature mid-infrared quantum cascade laser using a molecular sub-doppler reference. Opt. Lett. 37, 4811, https://doi.org/10.1364/ol.37.004811 (2012).
Gambetta, A. et al. Comb-assisted spectroscopy of CO2 absorption profiles in the near- and mid-infrared regions. Appl. Phys. B 109, 385–390, https://doi.org/10.1007/s00340-012-4947-3 (2012).
Jacquemart, D. et al. Infrared spectroscopy of CO2 isotopologues from 2200 to 7000 cm−1: I—Characterizing experimental uncertainties of positions and intensities. J. Quant. Spectrosc. Radiat. Transf. 113, 961–975, https://doi.org/10.1016/j.jqsrt.2012.02.020 (2012).
Lyulin, O. M. et al. Infrared spectroscopy of 17O- and 18O-enriched carbon dioxide in the 1700-8300 cm−1 wavenumber region. J. Quant. Spectrosc. Radiat. Transf. 113, 2167–2181, https://doi.org/10.1016/j.jqsrt.2012.06.028 (2012).
Galli, I. et al. Absolute frequency measurements of CO2 transitions at 4.3 μm with a comb-referenced quantum cascade laser. Mol. Phys. 111, 2041–2045, https://doi.org/10.1080/00268976.2013.782436 (2013).
Liao, C.-C., Lien, Y.-H., Wu, K.-Y., Lin, Y.-R. & Shy, J.-T. Widely tunable difference frequency generation source for high-precision mid-infrared spectroscopy. Opt. Expr. 21, 9238, https://doi.org/10.1364/oe.21.009238 (2013).
Lu, Y. et al. Line paremeters of the 782 nm band of CO2. Astrophys. J. 775, 71, https://doi.org/10.1088/0004-637X/775/1/71 (2013).
Petrova, T. M. et al. Measurements of 12C16O2 line parameters in the 8790-8860, 9340-9650 and 11,430-11,505 cm−1 wavenumber regions by means of Fourier transform spectroscopy. J. Quant. Spectrosc. Rad. Transf. 124, 21–27, https://doi.org/10.1016/j.jqsrt.2013.03.017 (2013).
Long, D. A., Truong, G.-W., Hodges, J. T. & Miller, C. E. Absolute 12C16O2 transition frequencies at the kHz-level from 1.6 to 7.8 μm. J. Quant. Spectrosc. Rad. Transf. 130, 112–115, https://doi.org/10.1016/j.jqsrt.2013.07.001 (2013).
Truong, G.-W. et al. Comb-linked, cavity ring-down spectroscopy for measurements of molecular transition frequencies at the kHz-level, J. Chem. Phys. 138. https://doi.org/10.1063/1.4792372 (2013)
Borkov, Y. G., Jacquemart, D., Lyulin, O. M., Tashkun, S. A. & Perevalov, V. I. Infrared spectroscopy of 17O- and 18O-enriched carbon dioxide: Line positions and intensities in the 3200–4700 cm−1 region. Global modeling of the line positions of 16O12C17O and 17O12C17O. J. Quant. Spectrosc. Radiat. Transf. 137, 57–76, https://doi.org/10.1016/j.jqsrt.2013.11.008 (2014).
Karlovets, E. V., Kassi, S., Tashkun, S. A., Perevalov, V. I. & Campargue, A. High sensitivity cavity ring down spectroscopy of carbon dioxide in the 1.19-1.26 μm region. J. Quant. Spectrosc. Radiat. Transf. 144, 137–153, https://doi.org/10.1016/j.jqsrt.2014.04.001 (2014).
Burkart, J. et al. Communication: Saturated CO2 absorption near 1.6 μm for kilohertz-accuracy transition frequencies. J. Chem. Phys. 142, 191103, https://doi.org/10.1063/1.4921557 (2015).
Borkov, Y. G., Jacquemart, D., Lyulin, O. M., Tashkun, S. A. & Perevalov, V. I. Infrared spectroscopy of 17O- and 18O-enriched carbon dioxide: Line positions and intensities in the 4681-5337 cm−1 region. J. Quant. Spectrosc. Radiat. Transf. 159, 1–10, https://doi.org/10.1016/j.jqsrt.2015.02.019 (2015).
Gatti, D. et al. Comb-locked cavity ring-down spectrometer, J. Chem. Phys. 142 https://doi.org/10.1063/1.4907939 (2015)
Lamouroux, J. et al. CO2 line-mixing database and software update and its tests in the 2.1 μm and 4.3 μm regions. J. Quant. Spectrosc. Radiat. Transf. 151, 88–96, https://doi.org/10.1016/j.jqsrt.2014.09.017 (2015).
Jacquemart, D., Borkov, Y. G., Lyulin, O. M., Tashkun, S. A. & Perevalov, V. I. Fourier transform spectroscopy of CO2 isotopologues at 1.6 μm: Line positions and intensities. J. Quant. Spectrosc. Radiat. Transf. 160, 1–9, https://doi.org/10.1016/j.jqsrt.2015.03.016 (2015).
Long, D. A., Wójtewicz, S., Miller, C. E. & Hodges, J. T. Frequency-agile, rapid scanning cavity ring-down spectroscopy (FARS-CRDS) measurements of the (30012) ← (00001) near-infrared carbon dioxide band. J. Quant. Spectrosc. Rad. Transf. 161, 35–40, https://doi.org/10.1016/j.jqsrt.2015.03.031 (2015).
Petrova, T. M. et al. Measurements of CO2 line parameters in the 9250–9500 cm−1 and 10,700–10,860 cm−1 regions. J. Quant. Spectrosc. Radiat. Transf. 164, 109–116, https://doi.org/10.1016/j.jqsrt.2015.06.001 (2015).
Tan, Y. et al. Cavity ring-down spectroscopy of CO2 overtone bands near 830 nm. J. Quant. Spectrosc. Rad. Transf. 165, 22–27, https://doi.org/10.1016/j.jqsrt.2015.06.010 (2015).
Benner, D. C. et al. Line parameters including temperature dependences of air- and self-broadened line shapes of 12C16O2: 2.06-μm region. J. Mol. Spectrosc. 326, 21–47, https://doi.org/10.1016/j.jms.2016.02.012 (2016).
Devi, V. M. et al. Line parameters including temperature dependences of self- and air-broadened line shapes of 12C16O2: 1.6-μm region. J. Quant. Spectrosc. Radiat. Transf. 177, 117–144, https://doi.org/10.1016/j.jqsrt.2015.12.020 (2016).
Vasilchenko, S. et al. The CO2 absorption spectrum in the 2.3 μm transparency window by high sensitivity CRDS: (I) Rovibrational lines. J. Quant. Spectrosc. Radiat. Transf. 184, 233–240, https://doi.org/10.1016/j.jqsrt.2016.07.002 (2016).
Guan, Y.-C. et al. Frequency measurements and molecular constants of the 12C\({}^{16}{\rm{O}}_{2}{[1{0}^{0}1,0{2}^{0}1]}_{{\rm{II}}}\leftarrow 0{0}^{0}0\) band near 2.7 μm. J. Mol. Spectrosc. 334, 26–30, https://doi.org/10.1016/j.jms.2017.03.011 (2017).
Kassi, S., Karlovets, E. V., Tashkun, S. A., Perevalov, V. I. & Campargue, A. Analysis and theoretical modeling of the 18O enriched carbon dioxide spectrum by CRDS near 1.35 μm: (I) 16O12C18O, 16O12C17O, 12C16O2 and 13C16O2. J. Quant. Spectrosc. Radiat. Transf. 187, 414–425, https://doi.org/10.1016/j.jqsrt.2016.09.002 (2017).
Čermák, P. et al. High sensitivity CRDS of CO2 in the 1.74 μm transparency window. A validation test for the spectroscopic databases. J. Quant. Spectrosc. Radiat. Transf. 207, 95–103, https://doi.org/10.1016/j.jqsrt.2017.12.018 (2018).
Karlovets, E. V. et al. Analysis and theoretical modeling of the 18O enriched carbon dioxide spectrum by CRDS near 1.74 μm. J. Quant. Spectrosc. Radiat. Transf. 217, 73–85, https://doi.org/10.1016/j.jqsrt.2018.05.017 (2018).
Gotti, R. et al. Comb-locked frequency-swept synthesizer for high precision broadband spectroscopy. Sci. Rep. 10, 2523, https://doi.org/10.1038/s41598-020-59398-1 (2020).
Karlovets, E. V., Kassi, S. & Campargue, A. High sensitivity CRDS of CO2 in the 1.18 μm transparency window. Validation tests of current spectroscopic databases. J. Quant. Spectrosc. Radiat. Transf. 247, 106942, https://doi.org/10.1016/j.jqsrt.2020.106942 (2020).
Lamperti, M. et al. Optical frequency metrology in the bending modes region, Commun. Phys. 3 https://doi.org/10.1038/s42005-020-00441-y (2020).
Reed, Z. D., Long, D. A., Fleurbaey, H. & Hodges, J. T. SI-traceable molecular transition frequency measurements at the 10−12 relative uncertain level. Optica 7, 1209–1220, https://doi.org/10.1364/OPTICA.395943 (2020).
Wu, H. et al. A well-isolated vibrational state of CO2 verified by near-infrared saturated spectroscopy with kHz accuracy. Phys. Chem. Chem. Phys. 22, 2841–2848, https://doi.org/10.1039/C9CP05121J (2020).
Predoi-Cross, A. & Buldyreva, J. Characterization of line mixing effects in the 11101 ← 00001 band of carbon dioxide for pressures up to 19 atm. J. Quant. Spectrosc. Radiat. Transf. 272, 107793, https://doi.org/10.1016/j.jqsrt.2021.107793 (2021).
Reed, Z. D., Drouin, B. J., Long, D. A. & Hodges, J. T. Molecular transition frequencies of CO2 near 1.6 μm with kHz-level uncertainties. J. Quant. Spectrosc. Rad. Transf. 271, 107681, https://doi.org/10.1016/j.jqsrt.2021.107681 (2021).
Marinina, A. A. et al. Absorption spectrum of carbon dioxide in the 4350-4550 cm−1 region. Atmos. Ocean. Opt. 35, 8–13, https://doi.org/10.1134/s1024856022010109 (2022).
Tan, Y. et al. Cavity-enhanced saturated absorption spectroscopy of the (30012) - (00001) band of 12C16O2. J. Chem. Phys. 156, 044201, https://doi.org/10.1063/5.0074713 (2022).
Chen, T. Y., Steinmetz, S. A., Patterson, B. D., Jasper, A. W. & Kliewer, C. J. Direct observation of coherence transfer and rotational-to-vibrational energy exchange in optically centrifuged CO2 super-rotors. Nat. Commun. 14, 3227, https://doi.org/10.1038/s41467-023-38873-z (2023).
Fleurbaey, H. et al. 12CO2 transition frequencies with kHz-accuracy by saturation spectroscopy in the 1.99-2.09 μm region. Phys. Chem. Chem. Phys. 25, 16319–16330, https://doi.org/10.1039/D3CP01603J (2023).
Lyulin, O. M., Solodov, A. M., Solodov, A. A., Petrova, T. M. & Perevalov, V. I. The absorption bands of 12C16O2 near 718 nm. J. Quant. Spectrosc. Rad. Transf. 303, 108595, https://doi.org/10.1016/j.jqsrt.2023.108595 (2023).
Birk, M., Röske, C. & Wagner, G. The pressure dependence of the experimentally-determined line intensity and continuum absorption of pure CO2 in the 1.6 μm region. J. Quant. Spectrosc. Rad. Transf. 324, 109055, https://doi.org/10.1016/j.jqsrt.2024.109055 (2024).
Jiang, S., Tan, Y., Liu, A.-W., Zhou, X.-G. & Hu, S.-M. Saturated cavity ring-down spectroscopy of 12C16O2 near 1.57 μm. Chin. J. Chem. Phys. 37, 13–18, https://doi.org/10.1063/1674-0068/cjcp2305046 (2024).
Ritter, M. E., DeSouza, S. A., Ogden, H. M., Michael, T. J. & Mullin, A. S. Transient IR spectroscopy of optically centrifuged CO2 (R186–R282) and collision dynamics for the J = 244 − 282 states. Faraday Discuss. 251, 140–159, https://doi.org/10.1039/D3FD00179B (2024).
Serdyukov, V. I., Sinitsa, L. N. & Vasil’chenko, S. S. Highly sensitive Fourier transform spectroscopy with LED sources. J. Mol. Spectrosc. 290, 13–17, https://doi.org/10.1016/j.jms.2013.06.004 (2013).
Amat, G. & Pimbert, M. On Fermi resonance in carbon dioxide. J. Mol. Spectrosc. 16, 278–290, https://doi.org/10.1016/0022-2852(65)90123-2 (1965).
Rothman, L. S. & Young, L. D. G. Infrared energy levels and intensities of carbon dioxide–II. J. Quant. Spectrosc. Radiat. Transf. 25, 505–524, https://doi.org/10.1016/0022-4073(81)90026-1 (1981).
Toth, R. A., Brown, L. R., Miller, C. E., Devi, V. M. & Benner, D. C. Spectroscopic database of CO2 line parameters: 4300 − 7000 cm−1. J. Quant. Spectrosc. Radiat. Transf. 109, 906–921, https://doi.org/10.1016/j.jqsrt.2007.12.004 (2008).
Furtenbacher, T. 626M24 dataset of 12C16O2 https://doi.org/10.17605/OSF.IO/9PZEW (2024).
Acknowledgements
The authors thank STFC for funding the UK–Jordan collaboration under the Newton Fund grant ST/T001429/1. JT acknowledges the support of the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme through Advance Grant number 883830. The work of A.G.C. and F.T. received support from NKFIH (grant number K138233).
Funding
Open access funding provided by Eötvös Loránd University.
Author information
Authors and Affiliations
Contributions
A.A.A.A., J.T., T.F., and A.G.C. designed the study, with important contributions from the further co-authors, mainly responsible for collecting measured data. A.A.A.A., J.T., T.F., S.N.Y. and A.G.C. checked different aspects of the datasets (transitions and energy levels). A.A.A.A., J.T., and A.G.C. wrote the original version of the manuscript. All of the authors have contributed to writing the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azzam, A.A.A., Alatoom, D., Abou Doud, B.M.J. et al. The 626M24 dataset of validated transitions and empirical rovibrational energy levels of 16O12C16O. Sci Data 12, 532 (2025). https://doi.org/10.1038/s41597-025-04755-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-025-04755-w