Abstract
Developmental Dysplasia of the Hip (DDH) stands as one of the preeminent hip disorders prevalent in pediatric orthopedics. Automated diagnostic instruments, driven by artificial intelligence methodologies, are capable of providing substantial assistance to clinicians in the diagnosis of DDH. We have developed a dataset designated as Multitasking DDH (MTDDH), which is composed of two sub-datasets. Dataset 1 encompasses 1,250 pelvic X-ray images, with annotations demarcating four discrete regions for the evaluation of pelvic X-ray quality, in tandem with eight pivotal points serving as support for DDH diagnosis. Dataset 2 contains 906 pelvic X-ray images, and each image has been annotated with eight key points for assisting in the diagnosis of DDH. Notably, MTDDH represents the pioneering dataset engineered for the comprehensive evaluation of pelvic X-ray quality while concurrently offering the most exhaustive set of eight key points to bolster DDH diagnosis, thus fulfilling the exigency for enhanced diagnostic precision. Ultimately, we presented the elaborate process of constructing the MTDDH and furnished a concise introduction regarding its application.
Similar content being viewed by others
Background & Summary
Developmental dysplasia of the hip (DDH), encompassing conditions such as acetabular dysplasia, subluxation, and dislocation of the hip joint, is a prevalent pediatric disorder1,2. Its incidence varies significantly across geographical regions and ethnicities, ranging widely from 0.9 per 1,000 to 76.1 per 1,000 live births3,4. Reports indicate that 76% of osteoarthritis of the hip in adults stems from childhood hip dysplasia, leading many individuals to undergo total joint replacement due to disabling pain and functional impairment before reaching the age of 505,6. In underdeveloped areas, inadequate access to early radiographic screening and technological resources often results in late diagnosis and suboptimal treatment outcomes for affected infants7. Early diagnosis and intervention in infancy for DDH can achieve near-normal hip joint development8, highlighting the critical importance of timely detection and accurate diagnosis in ensuring healthy growth and maintaining a high quality of life for these children. The ability to identify and manage DDH effectively during the pediatric years directly influences whether a child will grow up without disability and enjoy a full, active adulthood.
In infants and toddlers, particularly those with mild acetabular dysplasia, definitive diagnosis often requires imaging examinations. Once Secondary Ossification Centers (SOCs) emerge, typically after approximately 4 to 6 months of age, ultrasound effectiveness may be compromised due to obstruction by these centers. Consequently, the preferred imaging modality becomes anteroposterior (AP) pelvic radiography9. However, the cooperation of infants during X-ray procedures is often limited, coupled with substandard practices at some primary healthcare facilities, resulting in a persistently high rate of “non-standard” AP pelvic X-ray production (Fig. 1(b)). A “standard” AP pelvic X-ray is characterized by symmetrical obturator foramina bilaterally and a pelvis anterior plane parallel to the coronal plane as shown in Fig. 1(a)10. The quality of the AP pelvic X-ray—defined by whether it meets these standard criteria—directly impacts the accuracy of hip joint parameter measurements, which in turn critically affects the reliability of DDH diagnosis. The parameters obturator diameters, obturator height, and distance between symphysis and Hilgengreiner’s line can be feasibly measured on X-ray and employed in clinical practice to assess the acceptability of the pediatric pelvic radiograph prior to measurement of the acetabular index10. The primary classification systems used for pediatric DDH include the Tönnis classification11 and the International Hip Dysplasia Institute (IHDI) classification12. Both systems categorize DDH based on the position of key points within specific coordinate quadrants.
Figure 1 presents several illustrative scenarios. In Fig. 1(a,b), examples of AP pelvic radiographs are shown, with (a) conforming to the “standard” and (b) not. The non-conformance in (b) results from the patient’s failure to lie flat during the examination, causing an imbalance between the left and right sides and posing challenges for subsequent diagnoses. Figure 1(c) through (f) display varying degrees of DDH severity as classified by the IHDI.
Artificial intelligence (AI) has emerged as a powerful tool for assisting disease diagnosis and treatment. While keypoint detection and image segmentation technologies have been widely applied for automated analysis of various diseases13,14,15, their adoption in developmental dysplasia of the hip (DDH) remains limited.
The development of effective AI models requires comprehensive, high-quality datasets. Currently, there is a notable lack of well-annotated public datasets for pediatric hip dysplasia, particularly those designed to automate both quality assessment of anteroposterior (AP) pelvic radiographs and DDH diagnosis.
Several challenges hinder the creation of pediatric DDH datasets:
-
1.
Data acquisition from pediatric patients involves navigating complex ethical/legal requirements for consent while ensuring consistent, high-quality data collection.
-
2.
Engaging pediatric specialists for image annotation is cost-prohibitive due to their specialized expertise.
-
3.
The importance of automating both AP pelvic radiograph quality assessment and DDH diagnosis has been significantly underestimated, slowing progress in pediatric orthopedics.
To cultivate a model endowed with the capacity to accurately identify and precisely classify DDH, it is indispensable to possess a dataset that amalgamates the pivotal annotation information requisite for both pelvic X-ray quality control and DDH diagnosis tasks.
In a concerted effort to invigorate the development and application of AI technology within the realm of pediatric DDH research, we have meticulously constructed a bespoke dataset dedicated to the evaluation of pelvic X-ray quality and the AI-facilitated diagnosis of hip dysplasia in children. This has given rise to the Multitasking DDH (MTDDH) dataset, which is bifurcated into two constituent sub-datasets.
Dataset 1 contains 1,250 pelvic X-ray images, annotated with four regions of interest crucial for evaluating the quality of pediatric pelvic X-rays: the right obturator foramen (ROF), left obturator foramen (LOF), right ilium (RI), and left ilium (LI). Moreover, it includes annotations for eight key points essential for computational analysis of pediatric hip dysplasia: (1) right tri-radiate cartilage center (RTCC), (2) left tri-radiate cartilage center (LTCC), (3) right acetabulum superolateral margin (RASM), (4) left acetabulum superolateral margin (LASM), (5) right femoral head center (RFHC), (6) left femoral head center (LFHC), (7) right midpoint of the superior margin of the ossified femoral metaphysis (RMOFM), (8) left midpoint of the superior margin of the ossified femoral metaphasis (LMOFM). Dataset 2 comprises 906 pelvic X-ray images, each accompanied by annotation data for the same eight key points used for modeling to assist in DDH diagnosis, matching the annotations in Dataset 1. In the case of infants with incompletely developed femoral heads, only six key points are marked. Specifically, key points (5) and (6) are omitted from the annotation process. In Dataset 2, the 906 pelvic X-ray films adopt the same annotation strategy as that for the eight key points used in DDH auxiliary diagnosis in Dataset 1. In our previous study on DDH diagnostic models16, we utilized 1,398 pelvic radiographs without publicly releasing the dataset. Despite this limitation, the study attracted significant research interest, with numerous investigators contacting the corresponding author for data access. To better serve the research community’s need for comprehensive datasets, we have now carefully curated Dataset 2 by: (1) excluding 133 images from external centers present in the original collection, and (2) conducting rigorous quality control and optimization of the remaining 906 radiographs. This refined Dataset 2 is being released alongside Dataset 1 to maximize their collective research value.
Methods
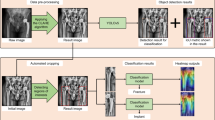
The development of our DDH dataset involved four main stages: data collection, preprocessing of the collected data, data labeling, and technical validation. The primary workflow of these activities is depicted in Fig. 2.
Data collection
This study has obtained the endorsement of the Medical Ethics Committee of Children’s Hospital, Zhejiang University of Medicine, as evidenced by the Approval Letter of IRB/EC (2023-IRB-0287-P-01), which also greenlit the public release of these data. Bearing in mind that this research involved a retrospective gathering of patients’ historical records and that all the data were anonymized, thus successfully eradicating any lurking threats of breaching patient privacy or detrimentally influencing their well-being, the obligation to solicit patients’ informed consent was duly waived. Every method adopted adhered stringently to the principles laid out in the Declaration of Helsinki. By leveraging technical means, we have expunged patient identifiers such as medical record numbers, names, and home addresses, thereby ensuring the utmost safeguarding of patient privacy. The pediatric DDH dataset originates from patients diagnosed with DDH at Children’s Hospital, Zhejiang University of Medicine between June 2017 and February 2019. The data types encompass anteroposterior pelvic X-rays along with corresponding personal demographic information (month’s age, gender). All enrolled patients had undergone X-ray examinations, with their images acquired utilizing state-of-the-art equipment from leading manufacturers such as Philips, GE, and Canon. The exposure dosage ranged from 1 to 2.5 µGym^2. For this study, X-ray images from these examinations were initially selected according to stringent inclusion and exclusion criteria. Each patient was provided with merely one pelvic X-ray image for enrollment.
The inclusion criteria for the study were as follows: (1) Patients must be at least 5 months or older but under 40 months of age. (2) Pelvic radiographs must adhere to established standard guidelines to ensure quality and consistency. (3) The primary reason for the visit should be a concern regarding potential hip dysplasia, indicated by the chief complaint being “evaluation for hip dysplasia.” (4) Only pre-treatment X-ray images of patients diagnosed with DDH were considered.
The criteria for data exclusion: (1) patients of hip dysplasia had been surgically treated; and (2) combined with other hip joint diseases, such as infection, femoral head Perthes disease, etc.
Standard pelvic radiograph requirements are as follows: (1) During the examination, the lower limbs are naturally straightened, the outside of the knees is flushed with the shoulders, the hips are slightly flexed, and the feet are taken in about 15°. (2) The size of the bilateral ilium and the obturator is basically symmetrical, the anterior and posterior edges of the acetabulum overlap, and the posterior margin of the acetabulum is not visible in x-ray image.
Data preprocessing
To address distinct tasks, two separate datasets were constructed based on differing annotation content, with each undergoing its own preprocessing steps. For Dataset 1: Utilizing RadiAnt DICOM viewer 2023.1, we converted the anteroposterior pelvic X-ray image data from Digital Imaging and Communications in Medicine (DICOM) format to JPEG format. A pelvic detection box with dimensions of 256 \(\times \) 192 was established to trim extraneous portions of the images. For Dataset 2: Anteroposterior pelvic radiographs were converted from DICOM format to PNG images using Python (version 3.6) and SimpleITK library (version 1.2.3). Images were resized to 1,333,800 pixels by keeping the original aspect ratio and padding zeroes on the shorter side. Resized images were further enhanced by applying window level and window width calculated by contrast-limited adaptive histogram equalization.
Data labeling
In this study, we performed two distinct annotation tasks: bone segmentation for quality assessment of anteroposterior pelvic radiographs, and anatomical keypoint detection for AI-assisted DDH diagnosis. The annotation was performed by a team of three pediatric orthopedic specialists: two junior surgeons (5 years of clinical experience each) and one senior surgeon (20 years of clinical experience). Our two-phase annotation protocol involved: (1) independent annotation and consensus-building by the junior surgeons, followed by (2) comprehensive quality control by the senior surgeon, who verified each annotation and mandated revisions when necessary. Complete methodological details regarding the 8 diagnostic keypoints for DDH evaluation and the 4 segmented bone regions for radiographic quality control are provided in the dedicated “Key Point Positioning” and “Bone Segmentation” sections.
Key point positioning
As illustrated in Fig. 3, the clinical assessment of DDH mainly relies on the IHDI and the Tönnis classification. The IHDI introduced a system for categorization based on the relative positioning of the center of the proximal femoral epiphysis in relation to the acetabulum. IHDI Classification: Hilgenreiner’s line (H) is delineated by connecting the lower borders of the iliac bones just above the bilateral Y-shaped growth plates. Perkins’ line (P) is constructed as a perpendicular line to Hilgenreiner’s line, passing through the outer upper rim of the acetabulum. The angle bisector of the inferior and lateral quadrant created by the intersection of lines H and P is designated as the D line. The midpoint of the proximal femoral metaphysis is utilized as a diagnostic reference point for DDH. The Tönnis classification is grounded in the relative location of the femoral head’s ossification center in comparison to the acetabulum: A line (SMA) is drawn connecting the superolateral margins of the acetabulum bilaterally. Perkins’ line (P) is a perpendicular line to the SMA, intersecting at the outer upper margin of the acetabulum, creating four quadrants. The position of the femoral head’s ossification center is employed as a diagnostic criterion for DDH. Clinically, physicians primarily refer to these two internationally recognized classification systems to facilitate the diagnosis of DDH, followed by the formulation of an appropriate treatment plan. As such, we have integrated both classification criteria to pinpoint eight pivotal landmarks that hold significant relevance under both systems. We used a keypoint annotation tool on a private platform called “Deepwise” (https://label.deepwise.com)to mark the 8 keypoints (RTCC, LTCC, RASM, LASM, RFHC, LFHC, RMOFM and LMOFM) on pelvic X-ray images as closely as possible according to human skeletal anatomy.
Radiographic classification systems for DDH. (a) Tönnis classification for DDH in children. The circle marks the femoral head ossification center. The SMA line connects the superior outer acetabular margins. The P line runs perpendicular to the SMA line through the outer acetabular edge. (b) IHDI classification for DDH in children. The circle indicates the proximal femoral metaphysis midpoint. The H line spans the inferior iliac borders above the triradiate cartilage. Line D bisects the inferior lateral quadrant formed by H and P lines.
Bone segmentation
A lack of significant pelvic inclination (within the sagittal plane) or rotation (within the horizontal plane) is a pivotal criterion in determining whether an anteroposterior pelvic X-ray conforms to “standard” specifications10. In normal human anatomy, the bilateral obturator foramina and ilia are characteristically distributed in a roughly symmetrical fashion. Utilizing the free annotation software Digimizer v6, equipped with features for image review, magnification, contrast improvement, and the fitting of circles and ellipses, experts meticulously traced the contours of the bilateral obturator foramina and the external margins of the iliac bones on anteroposterior pelvic radiographs. Pixels within the fitted area were then mapped to a binary pixel-wise segmentation mask. A comprehensive quality evaluation dataset specific to anteroposterior pelvic radiographs has been created. Building upon this resource, investigators have the opportunity to combine it with four pivotal indices: the obturator foramen rotation index, the obturator foramen area ratio, the iliac bone rotation index, and the iliac bone area ratio. This amalgamation facilitates the development of an artificial intelligence-driven quality evaluation model, designed to evaluate the symmetry and determine if such X-ray images meet the criteria for being considered “standard” in terms of their alignment and anatomical presentation.
Data Records
The MTDDH dataset16, designed for pelvic X-ray quality assessment and developmental dysplasia of the hip (DDH) diagnosis, is illustrated in Fig. 4 and publicly available via Science Data Bank. The dataset is divided into two subsets: Dataset 1 (focused on X-ray quality control and diagnostic keypoints) and Dataset 2 (structured for DDH auxiliary diagnosis in COCO format).
Dataset 1 contains two annotated subsets: (1) region segmentation data for X-ray quality evaluation and (2) eight anatomical keypoints for DDH diagnosis. The keypoints are organized into Training, Validation, and Test subfolders (JPG images), with JSON annotations provided for Training and Validation sets. The region segmentation data follows a similar structure but omits the Test subset. To ensure patient privacy, all filenames are randomly generated.
Dataset 2 adopts the COCO (Common Objects in Context) format, with annotations (JSON files) and images (PNG format) stored separately. The “coco” folder includes annotations for Train, Validation, and Test splits, while the corresponding images reside in a dedicated “png” folder. As in Dataset 1, all filenames are randomized to protect patient confidentiality.
Technical Validation
Two physicians independently annotated the dataset. Subsequently, a senior doctor meticulously examined the annotated content to ensure its precision. Dataset 1, consisting of 1,250 pelvic X-ray images, incorporated annotations for four regions that were essential for pelvic X-ray segmentation and eight key points that were pivotal for the auxiliary diagnosis of DDH. The data within Dataset 1 was partitioned into a Test set with 210 images, a Train set with 780 images, and a Validation set with 260 images. Dataset 2, which comprised 906 pelvic X-ray images, encompassed annotations for eight key points dedicated to DDH auxiliary diagnosis. The data of Dataset 2 was divided into 87 images in the Test set, 780 images in the Train set, and 88 images in the Validation set. Notably, all the data in Dataset 2, having served as a crucial foundation, had already been utilized in one of our previous works17. Table 1 details the specific quantity distribution of the two datasets by task type and subset type, while Table 2 presents the specific annotation details.
To intuitively depict the distribution of the enrolled patients, a cursory statistical analysis was conducted on their ages, genders, and other relevant aspects. The outcomes of this analysis are presented as follows. In Dataset 1, comprising 1,250 patients, 781 are female and 469 are male. The patients’ average monthly age is 14.57 months, with a median of 13 months and a variance of 65.66. Regarding Dataset 2, it contains 906 patients, among which 558 are female and 384 are male. The average monthly age of these patients is 14.32 months, with a median of 12 months and a variance of 64.82. The detailed breakdown is illustrated in Fig. 5.
In the context of evaluating the quality of pelvic X-rays, no pertinent clinical guidelines exist at present. Upon conducting extensive research, this study is set to introduce the first publicly accessible dataset designed for pelvic X-ray quality control. Drawing upon our extensive clinical experience and through numerous rounds of experimentation, we have devised a set of comparatively viable quantitative methodologies.
In the pursuit of accurate image symmetry analysis, we extracted the maximum transverse diameters and areas of both the obturator foramen and the iliac bone. Key notations were established: \({D}_{{Lt}}^{{ITD}}\) and \({D}_{{Rt}}^{{ITD}}\) precisely represented the maximum transverse extents of the left and right iliac bones respectively, while \({D}_{{Lt}}^{{OTD}}\) and \({D}_{{Rt}}^{{OTD}}\) were used to denote the corresponding measurements for the left and right obturator foramina. Moreover, the areas of the left and right iliac bones were defined as \({{a}}_{l}^{I}\) and \({a}_{r}^{I}\), and those of the left and right obturator foramina as \({a}_{l}^{O}\) and \({a}_{r}^{O}\).
Ilium transverse diameter
To calculate this, we first pinpointed the coordinates of pixel points flanking the minimum circumscribed rectangle of the ilium within the pelvis. For the left side, these coordinates were (\({x}_{l1}^{I}\), \({y}_{l1}^{I}\)) and (\({x}_{l2}^{I}\), \({y}_{l2}^{I}\)), and for the right side, (\({x}_{r1}^{I}\), \({y}_{r1}^{I}\)) and (\({x}_{r2}^{I}\), \({y}_{r2}^{I}\)). By subtracting the right abscissa values from the left ones, we obtained the crucial \({D}_{{Lt}}^{{ITD}}\) and \({D}_{{Rt}}^{{ITD}}\), as formulated below:
Obturator transverse diameter
A similar process was applied. We identified the coordinates of pixel points on either side of the minimum circumscribed rectangle of the obturator foramen. The left side coordinates were (\({x}_{l1}^{O}\), \({y}_{l1}^{O}\)) and (\({x}_{l2}^{O}\), \({y}_{l2}^{O}\)), and the right side were (\({x}_{r1}^{O}\), \({y}_{r1}^{O}\)) and (\({x}_{r2}^{O}\), \({y}_{r2}^{O}\)). Subsequently, subtracting the right abscissa values yielded the \({D}_{{Lt}}^{{OTD}}\) and \({D}_{{Rt}}^{{PTD}}\) :
Rotation indices and area ratios
The ilium and obturator rotation indices were computed to quantitatively assess potential asymmetries. The formulas were as follows:
The pixel sizes of the segmented regions of the iliac bone and obturator foramen (\({a}_{l}^{I}\),\(\,{a}_{r}^{I}\),\(\,{a}_{l}^{O}\),\(\,{a}_{r}^{O}\)) provided a direct reflection of the area magnitudes. The iliac bone rotation index, in particular, was derived by comparing the pixel sizes of the bilateral iliac bones:
Quality control standards
We established stringent internal quality evaluation baselines. For a “standard” anteroposterior pelvic X-ray, the following criteria must be satisfied:
The obturator foramen rotation index ((Rr1)) must fall within the range of 0.78–1.42.
The ratio of the areas of the bilateral obturator foramina should be confined to 0.77–1.46.
The iliac bone rotation index (Rr2) is required to be within 0.71–1.22.
The ratio of the areas of the bilateral iliac bones should remain within 0.71–1.22.
Despite the fact that our dataset has been meticulously annotated by three experienced clinical practitioners, thereby fulfilling the requirements of key point detection and segmentation tasks integral to both the DDH auxiliary diagnosis and pelvic X-ray quality control procedures, a number of challenges persist. Primarily, we do not claim to be the trailblazers in publicly disseminating a dataset designed specifically for DDH auxiliary diagnosis. A prior research endeavor culminated in the construction of a dataset comprising 10,000 pelvic X-ray images, which served as the inaugural public dataset for DDH diagnosis18. However, the access pathway to this dataset has currently become inoperative. In parallel, other investigations focusing on DDH diagnosis via X-rays have scarcely made their datasets publicly accessible, and moreover, the quantity of positive samples within these datasets is typically meager19,20. In contrast, our dataset has successfully mitigated this shortcoming by providing a substantial contribution. Furthermore, in adherence to the research methodology adopted by the aforementioned precedent study, our dataset undertakes the conversion of data from the original DICOM format. This transformation, while potentially leading to the partial forfeiture of information, has been subject to extensive deliberation among clinical experts. After comprehensive consultations, it has been ascertained that the conversion of image formats is not anticipated to exert a profound impact on the diagnostic outcome.
Usage Notes
The present dataset fulfills dual functions within the medical field. Firstly, it functions as a reliable resource for ensuring the quality of anteroposterior pelvic X-ray imaging. Given the strict postural demands for infants and toddlers, who frequently struggle to comply, non-standard anteroposterior pelvic X-ray images commonly emerge in clinical practice. Before diagnosing DDH, evaluating the standardization-that is, accurately assessing the quality-of these X-ray images is of the utmost significance. The acetabular index, a crucial parameter for diagnosing hip dysplasia, can be distorted by pelvic misalignment, especially in infants. Our aim is to provide a dataset that facilitates the exploration of the relationship between pelvic orientation and the acetabular index, identifying dependable predictors for pelvic orientation on plain radiographs.
Secondly, the dataset serves as a valuable medical asset for facilitating DDH diagnosis. The acetabular index, calculated using the Hilgenreiner method, is the most commonly and pivotally used metric for assessing acetabular development. However, the reproducibility of diagnostic measurements on anteroposterior pelvic X-rays for DDH is plagued by inaccuracies, leading to substantial measurement differences that impede reliable diagnoses and prompt referrals for many patients. Our effort is to offer a medical dataset to spur the development of an AI-powered diagnostic model for DDH. The eight annotated key points are fully compliant with the international DDH diagnostic criteria established by Tönnis and the IHDI, ensuring wide applicability.
Nevertheless, while this dataset reflects, to some extent, the natural occurrence and distribution of pediatric DDH, it has its limitations. For one thing, since the dataset includes patients older than five months, mostly evaluated via anteroposterior pelvic X-rays, there is a lack of data regarding neonates and infants under five months old, for whom ultrasound is the primary diagnostic tool. Our future plans involve filling this gap by incorporating ultrasound data to enhance the dataset. Additionally, the current dataset stems from a single medical center, and our strategy entails expanding it with multi-center data to improve its representativeness and applicability across different populations.
Code availability
The source code16 repositories for the segmentation algorithm and keypoint detection algorithm are publicly accessible at Science Data Bank, enabling reproducibility of the proposed medical image quality assessment and developmental dysplasia of the hip diagnosis framework.
References
Diaz-Lopez, R. A. et al. Impact of femoroacetabular impingement and dysplasia of the hip on hip joint sphericity. Hip International 30(2), 195–203, https://doi.org/10.1177/1120700019834295 (2020).
Schmitz, M. R., Murtha, A. S. & Clohisy, J. C. Developmental Dysplasia of the Hip in Adolescents and Young Adults. Journal of The American Academy of Orthopaedic Surgeons 28(3), 91–101, https://doi.org/10.5435/JAAOS-D-18-00533 (2020).
Studer, K. et al. Increase in late diagnosed developmental dysplasia of the hip in South Australia: risk factors, proposed solutions. Medical Journal of Australia 204(6), 240, https://doi.org/10.5694/mja15.01082 (2016).
St George, J. et al. Importance of early diagnosis for developmental dysplasia of the hip: A 5-year radiological outcome study comparing the effect of early and late diagnosis. Journal of Paediatrics and Child Health 57(1), 41–5, https://doi.org/10.1111/jpc.15111 (2021).
Schlung, J., Schrffman, S. & Chaturvedi, A. Top Ten Adult Manifestations of Childhood Hip Disorders An Up-To-Date Review for General Radiologists. Radiologic Clinics of North America 58(3), 529–+, https://doi.org/10.1016/j.rcl.2020.01.002 (2020).
Biedermann, R. et al. Results of universal ultrasound screening for developmental dysplasia of the hip A PROSPECTIVE FOLLOW-UP OF 28 092 CONSECUTIVE INFANTS. Bone & Joint Journal 100B(10), 1399–404, https://doi.org/10.1302/0301-620X.100B10.BJJ-2017-1539.R2 (2019).
Pollet, V., Percy, V. & Prior, H. J. Relative risk and incidence for developmental dysplasia of the hip. Journal of Pediatrics 181, 202–7, https://doi.org/10.1016/j.jpeds.2016.10.017 (2017).
Loder, R. T. & Skopelja, E. N. The epidemiology and demographics of hip dysplasia. ISRN Orthop 2011, 238607, https://doi.org/10.5402/2011/238607 (2011).
Barrera, C. A. et al. Imaging of developmental dysplasia of the hip: ultrasound, radiography and magnetic resonance imaging. Pediatric Radiology 49(12), 1652–68, https://doi.org/10.1007/s00247-019-04504-3 (2019).
Yang, Y. et al. How to judge pelvic malposition when assessing acetabular index in children? Three simple parameters can determine acceptability. Journal of Orthopaedic Surgery and Research 15(1), 12, https://doi.org/10.1186/s13018-020-1543-9 (2020).
Doski, J., Mosa, L. & Hassawi, Q. An Upgrade of the International Hip Dysplasia Institute Classification for Developmental Dysplasia of the Hip. Clinics in Orthopedic Surgery 14(1), 141–7, https://doi.org/10.4055/cios21075 (2022).
Pullen, W. M. et al. The Reliability of the Tonnis Grading System in Patients Undergoing Hip Preservation. American Journal of Sports Medicine 51(2), 476–80, https://doi.org/10.1177/03635465221147055 (2023).
Hassan, C. et al. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy A Systematic Review and Meta-analysis. Annals of Internal Medicine 176(9), 1209–+, https://doi.org/10.7326/M22-3678 (2023).
Xue, C. et al. AI-based differential diagnosis of dementia etiologies on multimodal data. Nature Medicine 30, 2977–89, https://doi.org/10.1038/s41591-024-03118-z (2024).
Wang, J. et al. Artificial intelligence enables precision diagnosis of cervical cytology grades and cervical cancer. Nature Communications 15(1), 4369, https://doi.org/10.1038/s41467-024-48705-3 (2024).
Qi G. Q. et al. The MTDDH dataset for quality evaluation of pelvic X-ray and diagnosis of developmental dysplasia of the hip [Internet]. Science Data Bank. https://doi.org/10.57760/sciencedb.24372 (2025).
Xu, W. et al. A Deep-Learning Aided Diagnostic System in Assessing Developmental Dysplasia of the Hip on Pediatric Pelvic Radiographs. Frontiers in Pediatrics 9, 785480, https://doi.org/10.3389/fped.2021.785480 (2022).
Liu, C. et al. Misshapen Pelvis Landmark Detection With Local-Global Feature Learning for Diagnosing Developmental Dysplasia of the Hip. IEEE Transactions on Medical Imaging 39(12), 3944–54, https://doi.org/10.1109/TMI.2020.3008382 (2020).
Den, H., Ito, J. & Kokaze, A. Diagnostic accuracy of a deep learning model using YOLOv5 for detecting developmental dysplasia of the hip on radiography images. Scientific Reports 13(1), 6693, https://doi.org/10.1038/s41598-023-33860-2 (2024).
Zhang, S.-C. et al. Clinical application of artificial intelligence-assisted diagnosis using anteroposterior pelvic radiographs in children with developmental dysplasia of the hip. Bone & Joint Journal 102B(11), 1574–81, https://doi.org/10.1302/0301-620X.102B11.BJJ-2020-0712.R2 (2020).
Acknowledgements
This study was supported by the National Key R&D Program of China (No. 2023YFC2706400) and the Zhejiang Province Research Project of Public Welfare Technology Application (No. LTGY23H260006).
Author information
Authors and Affiliations
Contributions
G.Q.Q. and X.F.J. were responsible for collecting patient data from hospitals, organizing related materials, and creating data sets. J.L. and R.J.J. were responsible for organizing the language and writing the text part of the article. X.X.L. and Z.X.S. were responsible for designing and drawing the graphs and figures of the article. C.J.Q. and Z.Z. were responsible for the experiments of the scientific validation part of the dataset, and the proofreading and revision of the article. Y.G.Z. coordinated the work of all parties and supervised all aspects of the process. G.Y. and G.Q.Z. provided important guidance on the writing of the article. In the end, all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, G., Jiao, X., Li, J. et al. A dataset for quality evaluation of pelvic X-ray and diagnosis of developmental dysplasia of the hip. Sci Data 12, 865 (2025). https://doi.org/10.1038/s41597-025-05146-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-025-05146-x