Abstract
Exendin-4 (EX-4), a glucagon-like peptide-1 (GLP-1) receptor agonist, has been shown to reduce food intake and to increase proopiomelanocortin (POMC) gene expression in the hypothalamus. In this study, we examined the potential neural mechanisms by which these effects occur. Male Sprague Dawley rats were implanted with a cannula in the third ventricle of the brain through which an inhibitor of phosphatidylinositol-3 kinase (PI3K) (wortmannin) was administered, and EX-4 or vehicle was administered via intraperitoneal (IP) injection. The activity of PI3K/protein kinase B (AKT) and insulin receptor substrate-1 (IRS-1) in the hypothalamic arcuate was determined. We found that EX-4 treatment significantly decreased food intake and body weight. However, there were almost no changes in food intake and body weight when wortmannin injection (into the third ventricle) occurred prior to EX-4 IP injection. EX-4 not only increased the activity of PI3K/AKT, but it also increased IRS-1 activity. These results show that EX-4 likely suppresses food intake due to its ability to enhance insulin signaling.
Similar content being viewed by others
Introduction
The glucagon-like peptide-1 (GLP-1) system was recently established as a target for type 2 diabetes (T2D) and obesity treatment1,2,3, as it improves blood glucose regulation, reduces food intake, and helps to manage body weight4, 5. Human and nonhuman animal experiments have shown that GLP-1 affects the central nervous system (CNS) to alter food intake and body weight regulation. In human studies, the GLP-1 receptor (GLP-1R) ligand greatly enhances insulin release6 and reduces appetite and energy intake7, when it is peripherally administrated. In animal experiments, either peripheral or central administration of GLP-1R suppressed food intake at least in part via CNS GLP-1R activation8,9,10. In addition, centrally injected GLP-1R antagonist exendin-(9–39) can attenuate the intake suppression effects of the GLP-1R agonist11, 12; centrally, viral knockdown of endogenous CNS GLP-1-producing preproglucagon (PPG) neurons causes hyperphagia and increased body weight13. Recently, a study by Rupprecht et al.14 showed that GLP-1R activation in the hindbrain suppressed food intake via a phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT)-dependent pathway, which started to elucidate the specific CNS GLP-1R-expressing nuclei and the intracellular signaling mechanisms through which food intake suppression occurs. However, additional studies are needed to identify the role(s) of CNS GLP-1R in food intake and energy expenditure.
Food intake and energy expenditure is mediated in part by neuropeptides expressed in neurons within nuclei of the mediobasal hypothalamus15, 16. Orexigenic neuropeptides, such as neuropeptide Y (NPY) and agouti-related protein (AgRP), increase food intake and body weight, while α-melanocyte-stimulating hormone (α-MSH), a product of proopiomelanocortin (POMC), is an anorexigneic neuropeptide that reduces food intake. α-MSH is expressed in neurons of the hypothalamic arcuate nucleus (ARC), which are perfectly positioned to integrate short-term satiety and long-term adiposity signals for energy homeostasis16, 17. PI3K/AKT signaling is thought to mediate leptin and insulin action in POMC and AgRP-expressing neurons18. It has been suggested that insulin helps to suppress the urge for food intake in the CNS via PI3K/AKT signaling19. Upstream to PI3K/AKT in the insulin pathway, the activities of the β-subunit of the insulin receptor and insulin receptor substrate-1 (IRS-1)20, 21 are elevated in response to the ratio of phospho-insulin receptor/total insulin receptor and phospho-IRS-1/total IRS-1.
Our previous study suggested that GLP-1R agonist exendin-4 (EX-4) reduced food intake and body weight by altering POMC gene expression in the hypothalamus of rats8. However, whether insulin signaling is involved in the mechanism by which EX-4 reduces food intake remains unknown. CNS GLP-1R signaling is involved in energy balance22, while PI3K/AKT signaling is also required in the regulation of energy expenditures within the hypothalamus23. In addition, PI3K/AKT signaling is both the direct and indirect upstream target of POMC gene expression24,25,26. These data suggest that the intake suppressive effects of GLP-1R require PI3K/AKT signaling regulation.
To test our hypothesis, intracerebroventricular (ICV) administration of a PI3K inhibitor via the third cerebral ventricle and intraperitoneal (IP) injection of EX-4 was used to explore the mechanisms by which peripheral administration of EX-4 reduces food intake in the hypothalamic ARC in vivo. We found that the effects of ICV wortmannin on EX-4-induced reduced food intake occurred by increasing ARC PI3K signaling.
Results
Low dose ICV wortmannin did not affect food intake
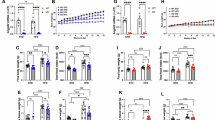
To identify the highest dose of wortmannin that inhibits PI3K in ARC without increasing food intake, we first conducted a dose-response test. Two-way ANOVA, Dose × Time, revealed a main effect of Dose [F(1,52) = 23.65, P < 0.001] and Time [F(3, 52) = 18.6, P < 0.001]. There was no significant Dose × Time effect [F(9, 52) = 0.93, P = 0.509]. The results are summarized in Fig. 1.
Food intake at 1, 2, 4, and 20 h after third ICV administration of different doses of wortmannin or vehicle. Food intake did not significantly increase in response to 0.5 ng or 1.5 ng wortmannin, but increased in response to 5 ng wortmannin. Data are represented as mean ± SEM (N = 4 for each group). In each panel, groups that share the same letter (eg. “a” and “ab”) are not significantly different from each other after obtaining a significant main effect by two-way ANOVA.
Administration of vehicle or 0.5 ng wortmannin resulted in similar food intake at every time point measured: 0–1 h (vehicle vs. 0.5 ng: 8.8 ± 0.6 g vs.9.3 ± 1.0 g, post-hoc P = 0.70), 0–2 h (vehicle vs. 0.5 ng: 9.8 ± 0.6 g vs.11.0 ± 1.1 g, post-hoc P = 0.37), 0–4 h (vehicle vs. 0.5 ng: 13.9 ± 0.4 g vs.14.5 ± 1.3 g, post-hoc P = 0.68) and 0–20 h (vehicle vs. 0.5 ng: 24.9 ± 0.8 g vs. 28.1 ± 1.4 g, post-hoc P = 0.24). While administration of 1.5 ng wortmannin appeared to decrease food intake at 0–1, 0–2, and 0–4 h and to increase food intake in 0–20 h, food intake was not significantly different compared to vehicle treatment [vehicle vs.1.5 ng: 0–1 h, 8.8 ± 0.6 g vs.6.7 ± 1.2 g, post-hoc P = 0.12; 0–2 h, 9.8 ± 0.6 g vs.7.7 ± 1.1 g, post-hoc P = 0.13; 0–4 h, 13.9 ± 0.4 g vs.11.0 ± 1.5 g, post-hoc P = 0.07; 0–20 h, 24.9 ± 0.8 g vs. 27.3 ± 1.7 g, post-hoc P = 0.38]. Finally, ICV administration of 5 ng wortmannin significantly increased food intake at 0–1 h (vehicle vs.5 ng: 8.8 ± 0.6 g vs.15.8 ± 0.7 g, post-hoc P < 0.001), 0–2 h (vehicle vs.5 ng: 9.8 ± 0.6 g vs.16.6 ± 0.7 g, P < 0.001), 0–4 h (vehicle vs.5 ng: 13.9 ± 0.4 g vs. 17.4 ± 0.4 g, P < 0.05), and 0–20 h (vehicle vs.5 ng: 24.9 ± 0.8 g vs.34.2 ± 2.9 g, P < 0.005) after a 4-hour fasting period.
In summary, 0.5 ng and 1.5 ng wortmannin injection did not significantly affect food intake, but 5ng wortmannin injection did.
Wortmannin reversed the suppression of food intake caused by intraperitoneal EX-4 injection
To examine the involvement of PI3K signaling in the food intake altering effects of EX-4, 1.5 ng wortmannin was administered via the ICV route prior to IP injection of EX-4 (3.2 µg/kg). Food intake was measured at 0–1, 0–2, 0–4, and 0–20 h after dark cycle initiation. Three-way repeated measures ANOVA (wortmannin × EX-4 × Time) revealed significant effects of wortmannin [F(1,44) = 10.8, P < 0.001], EX-4 [F(1,44) = 18.6, P < 0.001], Time [F(3,132) = 1913.9, P < 0.001], wortmannin × EX-4 [F(1, 44) = 4.4, P < 0.05] and EX-4 × Time [F(3, 132) = 4.7, P < 0.005] (Fig. 2A). The results indicated that peripheral EX-4 treatment combined with central vehicle (veh/EX-4) significantly reduced food intake as compared to the combinations of peripheral and central vehicle injections at each time point (veh/veh vs. veh/EX-4: 0–1 h, 4 ± 0.4 g vs. 2.4 ± 0.3 g, post-hoc P < 0.02; 0–2 h, 5.8 ± 0.4 g vs. 3.7 ± 0.3 g, post-hoc P = 0.013; 0–4 h,11.1 ± 0.7 g vs.7.0 ± 0.6 g, post-hoc P < 0.001; 0–20 h,26.0 ± 1.2 g vs. 22.7 ± 0.8 g, post-hoc P < 0.001). This reduction in food intake can be reversed by central 1.5 ng wortmannin injection prior to peripheral EX-4 treatment (wor/EX-4). Compared with the veh/EX-4 combination, wor/EX-4 resulted in significantly greater food intake (veh/EX-4 vs. wor/EX-4: 0–1 h, 2.4 ± 0.3 g vs.4.5 ± 0.3 g, P < 0.01; 0–2 h, 3.7 ± 0.3 g vs. 6.5 ± 0.4 g, P < 0.001; 0–4 h, 7.0 ± 0.6 g vs. 9.9 ± 0.5 g, P < 0.006; 0–20 h, 22.7 ± 0.8 g vs. 24.7 ± 0.7 g, P < 0.02]. Notably, there were no significant differences in food intake between the veh/veh and wor/EX-4 groups at any time point. Thus, EX-4 significantly decreased food intake, while wortmannin significantly attenuated the suppressive action of EX-4 on food intake at 0–1, 0–2, 0–4, and 0–20 h after 4 hours of fasting. Additionally, body weight was measured 24 hours after injections in these four groups. Two-way ANOVA (wortmannin × EX-4) revealed a significant effect of wortmannin × EX-4 [F(1,44) = 6.15, P = 0.017]. The wortmannin [F(1, 44) = 2.59, P = 0.114] or the EX-4 effect [F(1, 44) = 2.70, P = 0.108] was not significant. EX-4 treatment significantly reduced body weight (veh/EX-4 vs. veh/veh: −3.5 ± 1.1 g vs. veh/veh1.3 ± 1.5 g, post-hoc P < 0.005), and such an effect was attenuated by wortmannin (wor/EX-4 vs. veh/EX-4: 1.25 ± 1.1 g vs. −3.5 ± 1.1 g, post-hoc P < 0.005) (Fig. 2B).
Food intake at 1, 2, 4, and 20 h (A) and 24 h body weight change (B) after ICV administration of wortmannin or vehicle and intrapretoneal injection of EX-4 or vehicle. A, Food intake was significantly reduced by EX-4, and 1.5 ng wortmannin reversed this reduction. B, Body weight was reduced significantly by EX-4, and this effect was attenuated by 1.5 ng wortmannin. Data are represented as mean ± SEM (N = 8 for each group). In each panel, groups that share the same letter are not significantly different from each other after obtaining a significant main effect by three-way ANOVA (A) and two-way ANOVA (B).
The effect of EX-4 on PI3K/AKT was inhibited by wortmannin
Two-way ANOVA (Wortmannin × EX-4) revealed significant effects of wortmannin [F(1, 15) = 8.62, P < 0.01], EX-4 [F(1, 15) = 22.52, P < 0.001] and wortmannin × EX-4 [F(1,15) = 10.57, P < 0.007]. Third ICV wortmannin had no effect on AKT phosphorylation in ARC tissue at 3 h post-administration compared with central vehicle injection (wor/veh vs. veh/veh, P = 0.828). Intraperitoneal injection of EX-4 robustly increased phosphorylation of AKT 1 h post-administration compared with vehicle peripheral injection (veh/EX-4 vs. veh/veh, P < 0.001). This Ex-4 caused increase in AKT phosphorylation was inhibited by replacing the central vehicle with wortmannin (wor/EX-4 vs. veh/EX-4, P < 0.001). Together, these results indicate that the activity of PI3K/AKT was increased after peripheral injection of EX-4, and this change was reversed by a PI3K inhibitor (Fig. 3).
Activity of PI3K/AKT in the arcuate nucleus. EX-4 robustly increased phosphorylation of AKT in the ARC, and wortmannin inhibited this increase. In each panel, groups that share the same letter are not significantly different from each other after obtaining a significant main effect by two-way ANOVA. veh/veh: vehicle (5% DMSO in aCSF) third ICV and vehicle (water) IP; veh/EX-4: vehicle (5% DMSO in aCSF) third ICV and EX-4 IP; wor/veh: wortmannin third ICV and vehicle (water) IP; wor/EX-4: wortmannin third ICV and EX-4 IP.
The activity of IRS-1 was increased after EX-4 peripheral injection
To test the role of insulin signaling in the activation of PI3K/AKT in the ARC by peripheral injection of EX-4, protein expression of the β-subunit of the insulin receptor and IRS-1 were determined by Western blotting. ARC samples were from the veh/veh and veh/EX-4 groups. Both the insulin receptor and IRS-1 were activated significantly, evidenced by an increase in protein levels (veh/EX-4, P < 0.05). These findings indicated that peripheral EX-4 injection might enhance ARC insulin signaling via insulin receptor signaling to PI3K/AKT (Fig. 4).
Discussion
A previous study showed that EX-4 affects food intake and energy expenditure by activating GLP-1R27. In the present study, we found that EX-4, at a dose that induced anorexia, activated PI3K/AKT in the ARC. Furthermore, after injecting wortmannin into the third ventricle to block PI3K/AKT signaling26, the effects of EX-4 on food intake and body weight were significantly inhibited. Our results revealed that an insulin-IRS-1-PI3K/AKT-dependent pathway is involved in the mechanism through which GLP-1R in the ARC mediates food intake suppression.
PI3K encompasses a family of enzymes involved in cellular functions, such as cell growth, proliferation, differentiation, motility, and intracellular trafficking28. An essential downstream target of PI3K is AKT, which is activated via serine and threonine phosphorylation29. Current data suggest that hypothalamic PI3K signaling is important in the regulation of both energy balance and glucose metabolism23, 30. It has also been reported that PI3K increases the gene expression of POMC in the hypothalamic ARC25, 31, 32. Our previous study found that treatment with EX-4 increased POMC and decreased NPY expression in the hypothalamus of rats8. Meanwhile, there is evidence showing that GLP-1 receptors exist in the POMC/CART neurons, but not in NPY/AgRP neurons33. Previous studies have shown that EX-4 increases the activity of PI3K in peripheral tissues, such as liver, muscle, and pancreas34,35,36. There is possibility that EX-4 also increases the activity of PI3K in the POMC/CART neurons, which lead to increased expression of POMC, and resulted in decreased food intake.
In the current study, we aimed to determine whether the PI3K/AKT pathway also mediates the actions of EX-4 in the hypothalamus. We found that EX-4, at a dose of 3.2 μg/kg, significantly increased the phosphorylation of AKT in the ARC one hour after EX-4 administration, and at the same time food intake was notably decreased. If the phosphorylation of AKT is mediated by the PI3K signaling pathway, the increased phosphorylation of AKT induced by EX-4 should be reduced by pharmacological inhibition of the PI3K signaling pathway. Consistent with this hypothesis, injection of the PI3K inhibitor, wortmannin, into the third ventricle of the brain notably reduced the phosphorylation of AKT induced by EX-4, and the ability of EX-4 to decrease food intake was blocked. These data suggested that PI3K signaling mediates the downstream actions of EX-4 in ARC neurons. The study by Rupprecht et al.14 also suggested that PI3K/AKT pathway is involved in mediating suppressive effect of GLP-1R agonists on food intake, however, it was reported in their study that phosphorylation of AKT was suppressed when GLP-1 receptor was activated. One possible explanation for the discrepancy between our results is the different way of EX-4 injection (intraperitoneally vs. ICV). Furthermore, we focused on different region of the brain (ARC vs. hindbrain), and PI3K inhibitor was applied via 3rd ICV in the current study, but via 4th ICV in their study.
IRS-1 is an important component of insulin signaling, which is located upstream of PI3K/AKT20, 21. The activity of IRS-1 is increased after insulin binds with its receptor, which subsequently increases the activity of PI3K/AKT20, 21. It has also been reported that GLP-1 increased IRβ and IRS-1 in adipocytes37. To determine whether insulin signaling is involved in the activation of PI3K/AKT by EX-4, we assessed the activity of IRS-1 after peripheral EX-4 injection. Our results showed that phosphorylation of IRS-1 was markedly increased in ARC neurons one hour after EX-4 administration. These results provide evidence that EX-4 has a positive effect on IRS-1/PI3K/AKT signaling pathway in the ARC.
Schechter et al. previously described the production and secretion of insulin by fetal neurons in culture and demonstrated that neuronal synthesized insulin promoted neurofilamant distribution and axonal growth38. At the same time, exogenous insulin promoted neural differentiation and growth beyond central insulin39. The detailed molecular mechanisms by which EX-4 promotes insulin signaling in the ARC are still unclear. A possible explanation for the positive effect of EX-4 on IRS-1/PI3K/AKT signaling is that EX-4 promotes insulin secretion from neuronal cells or enhances insulin translocation through the blood-brain-barrier, which leads to higher brain insulin levels, and stronger activation of insulin signaling. However, there is also the possibility that EX-4 increases the activity of IRS-1 directly without the involvement of insulin. Further research is required to elucidate the full effects of EX-4 on IRS-1/PI3K/AKT signaling.
In summary, this study demonstrates a critical role of the PI3K/AKT pathway in regulating food intake in the ARC of rats and suggests that the effect of EX-4 in suppressing food intake is most likely due to its positive effect on IRS-1/PI3K/AKT signaling in the ARC.
Methods
Animals
Forty-eight adult male Sprague-Dawley rats (Harlan Industries, Frederick, MD) weighing 250–275 grams were individually housed under standard conditions (12:12 light/dark cycle, lights on at 00:00 h, 50–60% humidity). First, 16 rats were used for identifying the dose of ICV wortmannin that produced minimal effects on food intake. The remaining 32 rats were used for feeding tests. Rats were presented ad libitum access to standard rat chow (Teklad Global 18% Protein Rodent Diet, Harlan, Harlan Laboratories) and tap water except where noted. All animal procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University and conformed to the guidelines of the National Institutes of Health.
Drugs
EX-4 (7000921, Bachem Americas, Inc. Torrance, CA) was dissolved in sterile water for injections, and a dose of 3.2 μg/kg was chosen based on our previous study40. Wortmannin (W1628, Sigma Aldrich, Inc. Saint Louis, MO) was dissolved in 5% DMSO in artificial cerebrospinal fluid (aCSF) (3525, R&D system, Minneapolis, MN) for ICV injections.
Surgical procedures
At least one week after arrival, rats were anesthetized using a mixture of ketamine (90 mg/kg) and xylazine (2.7 mg/kg) and placed in a stereotaxic device (Kopf Instruments). A 23-gauge sterile guide cannula (Plastics One) was then implanted into the third ventricle (stereotaxic coordinates: −3 mm relative to the bregma, 0 mm lateral from the midline, and −8 mm ventral from the skull surface). Cannulae were attached to the skull by dental acrylic and jeweler’s screws. After 7 days of recovery, a third ventricle cannula was placed and verified by 30-minute water intake in response to administration of 10 ng (in 2 µl) angiotensin II. Only data from rats that drank at least 5 ml more water in response to angiotensin II than to vehicle (2 µl aCSF) were included in the analyses.
Intracerebroventricular delivery of drugs and food intake
The purpose of this experiment was to determine whether ICV administration of wortmannin could block or attenuate the food intake suppressive effect of IP EX-4 injection. Sixteen rats were randomly assigned to 4 groups, and named as group “veh”, “0.5 ng”, “1.5 ng”, “5 ng”, respectively. After 7 days of recovery following cannulation surgery, food was taken away 4 hours before dark cycle onset. Two hours before dark cycle onset, three doses of wortmannin, 0.5, 1.5, 5 ng (in 2 µl/injection) or vehicle, were delivered to the third ventricle with a micropump (StoeltingCo., Wood Dale, IL) over a 2-minute period. Food was returned at dark cycle onset, and food intake was measured at 1, 2, 4, and 20 hours post-food presentation. Three experimental doses of wortmannin were chosen based on previous reports27,28,29. Based on the results of this test, we selected a dose of 1.5 ng wortmannin to test its effect on EX-4-associated food intake reduction.
Similar procedures were used to examine the effects of 1.5 ng wortmannin on EX-4-induced food intake reduction. Thirty-two rats were randomly assigned to 4 groups, with 8 rats in each group, and named as group “veh/veh”, “veh/EX-4”, “wor/veh”, and “wor/EX-4”. On the day of drug administration, food was taken away 4 hours before dark cycle onset. Two hours prior to dark cycle onset, rats received ICV injections of the PI3K inhibitor wortmannin (2 μl, 1.5 ng) or vehicle (2 μl 5% DMSO in aCSF). Five minutes prior to dark cycle onset, rats were injected with EX-4 intraperitoneally at a dose of 3.2 μg/kg or vehicle (water at a volume of 1 ml/kg). When the dark cycle began, rats were provided with standard chow, and measurements of food intake were taken 1, 2, 4, and 20 hours post-food presentation.
Analysis of the protein levels of the β subunit of the insulin receptor and IRS-1 in the ARC by Western blotting
Seven days after the feeding test, PI3K signaling was examined after wortmannin and EX-4 administration. Food intake in response to wortmannin and EX-4 were measured using the previously described procedures. One hour after dark cycle onset, rats were sacrificed with rapid decapitation, and the brains were collected and flash frozen on dry ice and stored in −80 °C freezer until processing. Coronal hypothalamic brain sections (500 µm thick) were cut with a cryostat. Hypothalamic nuclei punches were then taken using a blunt 19-G needle. Punches for ARC were taken at −3.20mm relative to the bregma, and the tissue was immediately homogenized on ice in 50 µl tissue lysis buffer (Cell Lysis Reagent, Sigma Aldrich, St. Louis, MO) supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). After 1 hour, the homogenates were centrifuged (15 min, 12,000 rpm, 4 °C), and supernatants were retained for protein determination. A protein assay kit (Pierce Microplate BCA, Thermo Fisher Scientific Inc., Rockford, IL) was used to determine the protein content. Whole tissue (13.7–26.1 μg) extracts were separated on 10% Bis-Tris SDS-PAGE gels (Life Technologies, Carlsbad, CA), followed by electrophoretic transfer to polyvinylidene fluoride membranes. Membranes were incubated with primary antibodies overnight at 4 °C and with secondary antibodies, and then they were conjugated to alkaline phosphatase at room temperature for 1 hour. Imaging was performed using a Typhoon FLA9500 imager (GE Healthcare), and densitometry was analyzed using Image J software. Primary antibodies for AKT, the phosphorylated site of Ser473 on AKT, IR, the phosphorylated site of Tyr1345 on IR, IRS-1, the phosphorylated site of Ser307 on IRS-1, and the secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The primary antibody against β-actin was purchased from Santa Cruz (Santa Cruz, CA, USA).
Statistical analysis
All data are presented as mean ± SEM. All behavioral parameters were analyzed by two-way or three-way repeated measures ANOVA followed by a post-hoc Fisher LSD test as appropriate or Student t test where only two conditions were compared. Data from Western blots were analyzed by two-way ANOVA. All statistical analyses were conducted using Statistica (version 7.1, Tulsa, OK). Differences were considered significant at P ≤ 0.05.
References
Inoue, K. et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovascular diabetology 10, 109, doi:10.1186/1475-2840-10-109 (2011).
Gupta, N. A. et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology (Baltimore, Md.) 51, 1584–1592, doi:10.1002/hep.23569 (2010).
Buse, J. B. et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes care 33, 1300–1303, doi:10.2337/dc09-2260 (2010).
van Bloemendaal, L., Ten Kulve, J. S., la Fleur, S. E., Ijzerman, R. G. & Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. The Journal of endocrinology 221, T1–16, doi:10.1530/joe-13-0414 (2014).
Skibicka, K. P. The central GLP-1: implications for food and drug reward. Frontiers in neuroscience 7, 181, doi:10.3389/fnins.2013.00181 (2013).
Kreymann, B., Williams, G., Ghatei, M. A. & Bloom, S. R. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet (London, England) 2, 1300–1304 (1987).
Flint, A., Kapitza, C. & Zdravkovic, M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes, obesity & metabolism 15, 958–962, doi:10.1111/dom.12108 (2013).
Yang, Y., Moghadam, A. A., Cordner, Z. A., Liang, N. C. & Moran, T. H. Long term exendin-4 treatment reduces food intake and body weight and alters expression of brain homeostatic and reward markers. Endocrinology 155, 3473–3483, doi:10.1210/en.2014-1052 (2014).
Barrera, J. G., D’Alessio, D. A., Drucker, D. J., Woods, S. C. & Seeley, R. J. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 58, 2820–2827, doi:10.2337/db09-0281 (2009).
Dalvi, P. S., Nazarians-Armavil, A., Purser, M. J. & Belsham, D. D. Glucagon-like peptide-1 receptor agonist, exendin-4, regulates feeding-associated neuropeptides in hypothalamic neurons in vivo and in vitro. Endocrinology 153, 2208–2222, doi:10.1210/en.2011-1795 (2012).
Kanoski, S. E., Fortin, S. M., Arnold, M., Grill, H. J. & Hayes, M. R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152, 3103–3112, doi:10.1210/en.2011-0174 (2011).
Williams, D. L., Baskin, D. G. & Schwartz, M. W. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150, 1680–1687, doi:10.1210/en.2008-1045 (2009).
Barrera, J. G. et al. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31, 3904–3913, doi:10.1523/jneurosci.2212-10.2011 (2011).
Rupprecht, L. E. et al. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. American journal of physiology. Endocrinology and metabolism 305, E751–759, doi:10.1152/ajpendo.00367.2013 (2013).
Horvath, T. L. Synaptic plasticity in energy balance regulation. Obesity (Silver Spring, Md.) 14(Suppl 5), 228s–233s, doi:10.1038/oby.2006.314 (2006).
Schwartz, M. W., Woods, S. C., Porte, D. Jr., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671, doi:10.1038/35007534 (2000).
Myers, M. G. Jr., Leibel, R. L., Seeley, R. J. & Schwartz, M. W. Obesity and leptin resistance: distinguishing cause from effect. Trends in endocrinology and metabolism: TEM 21, 643–651, doi:10.1016/j.tem.2010.08.002 (2010).
Varela, L. & Horvath, T. L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO reports 13, 1079–1086, doi:10.1038/embor.2012.174 (2012).
Niswender, K. D. et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52, 227–231 (2003).
Paz, K. et al. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. The Journal of biological chemistry 274, 28816–28822 (1999).
Gual, P., Le Marchand-Brustel, Y. & Tanti, J. F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87, 99–109, doi:10.1016/j.biochi.2004.10.019 (2005).
Lockie, S. H. Glucagon-like peptide-1 receptor in the brain: role in neuroendocrine control of energy metabolism and treatment target for obesity. Journal of neuroendocrinology 25, 597–604, doi:10.1111/jne.12039 (2013).
Xu, Y. et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab 12, 88–95, doi:10.1016/j.cmet.2010.05.002 (2010).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484, doi:10.1038/35078085 (2001).
Hill, J. W. et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of clinical investigation 118, 1796–1805, doi:10.1172/jci32964 (2008).
Rahmouni, K. et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. The Journal of clinical investigation 114, 652–658, doi:10.1172/jci21737 (2004).
Mul, J. D. et al. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. American journal of physiology. Regulatory, integrative and comparative physiology 305, R68–77, doi:10.1152/ajpregu.00588.2012 (2013).
Davis, W. J., Lehmann, P. Z. & Li, W. Nuclear PI3K signaling in cell growth and tumorigenesis. Frontiers in cell and developmental biology 3, 24, doi:10.3389/fcell.2015.00024 (2015).
Fayard, E., Xue, G., Parcellier, A., Bozulic, L. & Hemmings, B. A. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Current topics in microbiology and immunology 346, 31–56, doi:10.1007/82_2010_58 (2010).
Hill, J. W. et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 150, 4874–4882, doi:10.1210/en.2009-0454 (2009).
Xu, A. W. et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. The Journal of clinical investigation 115, 951–958, doi:10.1172/jci24301 (2005).
Williams, K. W. et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30, 2472–2479, doi:10.1523/jneurosci.3118-09.2010 (2010).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. The Journal of clinical investigation 124, 4473–4488, doi:10.1172/jci75276 (2014).
Favaro, E. et al. The ghrelin gene products and exendin-4 promote survival of human pancreatic islet endothelial cells in hyperglycaemic conditions, through phosphoinositide 3-kinase/Akt, extracellular signal-related kinase (ERK)1/2 and cAMP/protein kinase A (PKA) signalling pathways. Diabetologia 55, 1058–1070, doi:10.1007/s00125-011-2423-y (2012).
Moreno, P. et al. Normalizing action of exendin-4 and GLP-1 in the glucose metabolism of extrapancreatic tissues in insulin-resistant and type 2 diabetic states. Journal of molecular endocrinology 48, 37–47, doi:10.1530/jme-11-0127 (2012).
Erdogdu, O., Nathanson, D., Sjoholm, A., Nystrom, T. & Zhang, Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Molecular and cellular endocrinology 325, 26–35, doi:10.1016/j.mce.2010.04.022 (2010).
Gao, H. et al. GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3-L1 adipocytes. Endocrine 32, 90–95, doi:10.1007/s12020-007-9011-4 (2007).
Schechter, R. & Abboud, M. Neuronal synthesized insulin roles on neural differentiation within fetal rat neuron cell cultures. Brain research. Developmental brain research 127, 41–49 (2001).
Liu, J., Speder, P. & Brand, A. H. Control of brain development and homeostasis by local and systemic insulin signalling. Diabetes, obesity & metabolism 16(Suppl 1), 16–20, doi:10.1111/dom.12337 (2014).
Liang, N. C., Bello, N. T. & Moran, T. H. Additive feeding inhibitory and aversive effects of naltrexone and exendin-4 combinations. International journal of obesity (2005) 37, 272–278, doi:10.1038/ijo.2012.16 (2013).
Acknowledgements
We thank Dr. Yada Treesukosol, Gretha Boersma, and Lin Li for their technical advice and assistance with this study. We would also like to thank the Duoease Scientific Service Center for their language editing services. This work was supported by an Overseas Returnee Research Funding of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (2201101266 to Y.Y.); an Natural Science Foundation of China (NSFC) (81670754 to Y.Y.; 81500636 to D.M.); National Foundation of Science and Technology Department of Hubei Province(ZRMS2016000185 to Y.Y.), and a funding from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (DK-19302 to T.H.M.).
Author information
Authors and Affiliations
Contributions
N.L. and T.M. contributed to the study design; Y.Y. contributed to study design and conduction; P.C. and Z.C. contributed to animal experiments; W.S. and Y.Y. contributed to Western blots; W.X. and D.M. contributed to Western blots and statistical analysis.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Choi, P.P., Smith, W.W. et al. Exendin-4 reduces food intake via the PI3K/AKT signaling pathway in the hypothalamus. Sci Rep 7, 6936 (2017). https://doi.org/10.1038/s41598-017-06951-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06951-0
This article is cited by
-
Gestational saccharin consumption disrupts gut-brain axis glucose homeostasis control in adolescent offspring rats in a sex-dependent manner
Biology of Sex Differences (2025)
-
Inhibition of ANGPTL8 protects against diabetes-associated cognitive dysfunction by reducing synaptic loss via the PirB signaling pathway
Journal of Neuroinflammation (2024)
-
Sleeve Gastrectomy Improves High-Fat Diet–Associated Hepatic Steatosis Independent of the Glucagon-like-Petpide-1 Receptor in Rats
Journal of Gastrointestinal Surgery (2022)
-
Exendin-4 improves long-term potentiation and neuronal dendritic growth in vivo and in vitro obesity condition
Scientific Reports (2021)
-
Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes
Nature Communications (2020)