Abstract
Agricultural production combined with planting and breeding, which can reduce chemical fertilizer and pesticide applications, reduce losses due to natural disasters, and improve the output and quality of agricultural products, is an important way to achieve green, circular and efficient production. To assess effects on soil bacterial community structure, a vegetable-eel-earthworm integrated planting and breeding platform (VEE-IPBP) combined with experiment planting was established at Chongming Island, Shanghai and compared to traditional planting. High-throughput sequencing to reveal soil bacterial community structure was performed on samples collected at 0, 3 and 6 years after implementation of the two models. Over time, the Shannon index first increased and then decreased in the VEE-IPBP system and was reduced by 3.2% compared to the traditional planting (In the same time and space scale, the single-degree planting method of dryland vegetables under mechanical cultivation is adopted) (p < 0.05). In contrast, Chao and Ace indices were increased by 2.4% and 3.2%. Thus, soil bacterial diversity was markedly different in the two planting models. The abundance of Proteus, Cyanophyta and Cyanophyta in soil increased after 6 years, and the proportion of Lysinibacillus increased significantly, contributing to improvement in soil disease resistance. Redundancy analysis (RDA) showed that the soil pH and water content were the main factors influencing the change in soil bacterial community structure in the two planting models, and the dominant species of soil bacteria were Lysobacter and Bacillus.
Similar content being viewed by others
Introduction
Integrated planting and breeding is a circular eco-agricultural production approach that takes full advantage of ecological principles to achieve reasonable systematic allocation for matter–energy conversion. Indeed, integrated planting and breeding platforms (IPBPs) can help to increase agricultural resource utilization efficiency, protect agro-ecological environments and promote green agricultural development. IPBPs have been used extensively worldwide for some time, and there are a wide variety of IPBPs with varied functions. The economic, ecological and social benefits due to the implementation of IPBPs have recently been studied extensively1,2,3,4. In various countries and territories in Europe and the United States, grain production has been integrated with livestock and poultry breeding, e.g., forage-crop-cow, grain-vegetable-pig and rice-mushroom-goose IPBPs5,6. Research has shown that at a suitable scale, livestock and poultry breeding combined with grain and vegetation production is advantageous in that livestock and poultry excrement can be returned to the field after fermentation and composting. The effects on soil include increasing the nutrient content (e.g., organic matter (OM)), improving the physical structure and aeration, balancing the pH and significantly enhancing bacterial diversity. Furthermore, some countries in Asia have established systems by integrating aquaculture with traditional agricultural production (integrated agro-aquaculture), also with relatively high resource utilization efficiency and system productivity7,8. For example, rice-fish symbiotic systems have had the following impacts: increased rice production per unit area by 5–15%, organic fertilizer utilization efficiency, soil microbial populations and activities, and soil respiration; improved rice quality traits and soil physical and chemical properties; reduced chemical fertilizer and pesticide application, pests and diseases, and accumulation of harmful reducing substances. Overall, these changes alter the soil microbial community structure9,10.
An integrated model of vegetables, eels and earthworms (that is, VEE-IPBP) constitutes a farmland ecosystem comprising dryland vegetables, soil animals and benthic aquatic animals in a habitat in which water and drought coexist11,12. It is reported that a farmland ecosystem in which wet and dry fields coexist can increase yield per unit area by more than 50%, reduce economic losses from floods, pests and diseases by more than 30% and achieve 100% recycling and reuse of agricultural crop wastes (e.g., stalks)13. Most research to date on 3D VEE-IPBP has focused on reducing pesticide and chemical fertilizer application and greenhouse gas emissions and on increasing soil nutrient utilization efficiency, crop output, agricultural product quality, and use of agricultural crop wastes (e.g., stalks). However, studies on soil microbial diversity under VEE-IPBP have rarely been reported. Thus, the mechanism by which VEE-IPBP affects the soil microbial community structure remains unclear. Soil microbes are not only biotic agents responsible for soil formation but are also important components of the soil ecosystem and play a pivotal role in humus formation, OM decomposition and soil nutrient cycling and transformation14,15. With specific functions in soil matter transformation and energy flow, various physiological groups of soil microbes produce an active pool of plant nutrient elements16,17, and their presence and activity affect soil fertility and crop nutrient supply18. In fact, soil microbial diversity can reflect, with high sensitivity, the functional evolution of an ecosystem as well as environmental stress19,20,21, and it is the basis for sustainable farmland ecosystem development22. Therefore, by studying the species, communities and functional diversity of soil microbes in regional farmland, we can provide a basis for evaluating the rationality of planting systems in a certain area.
In this study, the multi-year effects of VEE-IPBP on soil microbial community structure and diversity were analysed using Illumina high-throughput sequencing technology, with the goal of providing basic parameters for improving VEE-IPBP management and control measures as well as a scientific basis for increasing soil fertility and reasonably and sustainably utilizing cultivated land resources.
Results and Discussion
Effects of IPBP on soil bacterial diversity indices
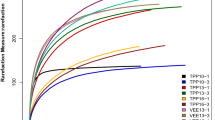
Mean Shannon index values of 6.08, 6.45, 6.44, 6.54 and 6.23 were obtained for soil samples TPP10, TPP13, TPP16, VEE13 and VEE16, respectively. These results indicate that diversity in the TPP-treated plots increased with time; in contrast, Shannon index values for VEE-IPBP-treated soils first increased and then decreased. Three years after implementation of the two planting systems, Shannon index values for soil in VEE-IPBP-treated plots (i.e., soil sample VEE13) were significantly higher than those in TPP-treated plots (i.e., soil sample TPP13). Although Shannon index values for VEE-IPBP-treated plots (i.e., soil sample VEE16) were significantly lower than those for soil in the TPP-treated plots (i.e., soil sample TPP16) (p < 0.05) after 6 years, that of the soil in both VEE-IPBP- and TPP-treated plots was higher than that of the basic soil (i.e., soil sample TPP10, collected before the beginning of the experiment). Species richness indices for the soil in plots under the two planting systems were also compared. Mean Chao index values for soil samples TPP10, TPP13, TPP16, VEE13 and VEE16 were 2,578, 3,027, 3,064, 3,133 and 3,138, respectively, and mean ACE index values for these samples were 2,583, 3,028, 3,062, 3,118 and 3,159, respectively (p < 0.05). Both indices increased as with time under the planting systems. In addition, soil bacterial species richness was higher in VEE-IPBP-treated plots than in TPP-treated plots (Table 1).
Currently, opinions differ with regard to whether earthworms promote or inhibit bacterial biomass and diversity. Most studies have concluded that as a result of their activities and foraging, earthworms increase soil aeration, with the enzymes in their intestinal secretions creating an environment that is favourable for increasing the soil bacterial biomass and diversity23,24,25,26. However, a large number of studies have also found that after passing through the intestines of earthworms, no change or significant increase in bacterial biomass occurs. Moreover, due to their feeding habits, burrow compression or surface secretions, a decrease in bacterial diversity or activity may even occur for some species of earthworm27,28,29,30,31. The results obtained in this study showed that compared to TPP, an increase in soil bacterial richness but a decrease in soil bacterial diversity occurred in VEE-IPBP-treated plots. This result may be due to a certain amount of water remaining in the ditches in VEE-IPBP-treated plots throughout the year, resulting in a relatively high soil moisture content. This directly affects bacterial activity in the intestinal and body-surface secretions of Pheretima guillemi earthworms or indirectly influences bacterial diversity by affecting the form, ratio and content of soil nutrients. Another possible reason is that by burrowing in the soil or feeding on soil and aquatic animals, the introduction of swamp eels might have affected soil bacterial richness. Regardless, the effects of swamp eels on soil microbes require further validation due to the current lack of research on this subject.
UniFrac-based principal coordinate analysis (PCoA) can visually display similarities and differences in microbial evolution between different environmental samples32. Figure 1 shows the results of Bray-Curtis PCoA comparing changes occurring in the two planting systems from an evolutionary perspective. As indicated in Fig. 1, the different planting systems and durations can be satisfactorily distinguished, which also indicates that the presence and activities of earthworms and swamp eels significantly affected soil microbial diversity. However, determination of the specific mechanism involved requires further research.
Effects of planting systems on soil bacterial community structure
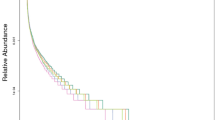
The bacteria in the soil samples from the plots subjected to various treatments were grouped at the phylum level. Based on the abundance of sequences contained in different operational taxonomic units (OTUs) in soil samples from plots subjected to different treatments, a community structure histogram was produced after clustering (Fig. 2A), revealing differences in bacterial community structure and relative abundance at the phylum level. A total of 12 bacterial phyla with abundances greater than 1% were found in soil samples collected from TPP- and VEE-IPBP-treated plots, namely, Proteobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, Actinobacteria, Planctomycetes, Gemmatimonadetes, Nitrospirae, Firmicutes, Latescibacteria, Thaumarchaeota and Cyanobacteria. Proteobacteria and Acidobacteria accounted for more than 60% of all phyla. An increase in the abundance of Proteobacteria, Firmicutes and Cyanobacteria was observed in VEE-IPBP-treated plots (i.e., soil sample VEE16) at 6 years after implementation of the planting systems. The majority of Proteobacteria and Firmicutes bacteria are facultative or obligate anaerobes and heterotrophs. Bacteria of the phylum Cyanobacteria are photosynthetic bacteria of the kingdom Eubacteria. The presence of ditches, which contained a certain amount of water throughout the year, in VEE-IPBP-treated plots may have created a suitable living environment for bacteria of the phylum Cyanobacteria, which showed increased abundance. Some researchers believe that although the environment in the digestive tracts of earthworms can help increase bacterial biomass, earthworms also feed on bacteria, resulting in a decrease in the population of certain bacterial phyla and an increase in anaerobic bacteria. This in turn results in a change in the composition of the bacterial communities in earthworm faeces, thereby affecting soil bacterial community composition33.
To further understand the functions of the bacteria present, the phyla in the soil samples collected from plots subjected to various treatments were clustered at the genus level. As indicated above, after clustering, a community structure histogram was produced based on the abundance of sequences contained in different OTUs (Fig. 2B). Genera with abundance ranked in the top five included Subgroup 6_norank, Nitrosomonadaceae_uncultured, Anaerolineaceae_uncultured, Gemmatimonadaceae_uncultured and Lysobacter. The abundance of Lysobacter in VEE-IPBP-treated plots had increased significantly at 6 years after implementation of the planting systems. Some researchers have found that Lysobacter bacteria can produce myxin34, and according to current research, most bacteria of this genus exert antagonistic effects toward pathogenic fungi, gram-positive (G+) bacteria, nematodes and green algae35. The sudden increase in the population of Lysobacter in the soil of VEE-IPBP-treated plots might be due to the “favourable” environment (suitable moisture content, neutral pH and high amino acid, low molecular weight sugar and fatty acid contents) of the earthworm intestines for growth36, possibly awakening dormant or inactive Lysobacter bacteria in the soil.
Differences between soil bacterial community groups
LEfSe, an algorithm for high-dimensional biomarker discovery and explanation, can determine the genomic functions between biotic conditions using two, or more than two, different characteristics. We used linear discriminant analysis (LDA) to distinguish differences in bacterial groups between the two planting systems and years37. The results (Fig. 3A) showed 41 communities with an LDA score greater than 4 (Fig. 3B). The distribution of communities with phylogenetic differences in soil samples collected from plots subjected to five different treatments was as follows: 10 at the phylum level, 11 at the class level, 10 at the order level, 7 at the family level and 3 at the genus level. At the phylum level, 3 phylogenies of bacteria in soil sample TPP10 (i.e., basic soil sample) exhibited relatively high relative abundance, namely, Acidobacteria, Chloroflexi and Gemmatimonadetes. After 3 years, Planctomycetes, Nitrospirae and Latescibacteria showed relatively high relative abundance in TPP-treated plots (i.e., soil sample TPP13); after 6 years, Bacteroidetes became the phylogeny with relatively high relative abundance (i.e., soil sample TPP16). Many species of Bacteroidetes reside in the intestines of humans and animals; most are pathogenic and be shed in faeces. In this study, bacteria of the phylum Bacteroidetes in the soil might have originated from the organic fertilizer applied, remaining and gradually becoming the dominant bacteria38. However, soil in VEE-IPBP-treated plots was notable for the presence and activities of earthworms and swamp eels, which adjusted soil bacterial community composition indirectly by affecting physical indices of the soil (nutrient content, element ratio, volume weight, porosity and pH) or directly by feeding on bacteria, such as those of Bacteroidetes, or providing a suitable growth environment for Proteobacteria, Actinobacteria and Firmicutes. Liu et al.39 and Liu et al.40 have found that soil environments with a high moisture content are more suitable for bacteria of the phylum Proteobacteria. In the present study, we found a relatively high relative abundance of Actinobacteria in the soil of VEE-IPBP-treated plots after 3 years (i.e., soil sample VEE13), with Proteobacteria and Firmicutes showing high relative abundance after 6 years (i.e., soil sample VEE16).
Redundant analysis of microbial communities and soil environmental factors
Correlations between soil bacterial community structure in VEE-IPBP- and TPP-treated plots and the physical and chemical properties of soil were analysed (Table 2), with explanatory power of 90.70% for all physical and chemical property variables for the different planting systems and durations (Fig. 4). Changes in the 10 main bacterial genera are most significantly correlated to the available potassium (AK) content of the soil, followed by the total phosphorous (TP), total nitrogen (TN), moisture and available N (AN) contents. Each planting systems caused changes in soil nutrients, thereby affecting soil bacterial communities. At the beginning of and at 3 years after implementation of VEE-IPBP, Nocardioides, Haliangium, Gaiella and Nitrospira were the main bacterial genera due to significant effects on TP, TK and TN contents. As the planting duration extended, each planting systems significantly affected soil physical and chemical properties as well as bacterial genera. Because of strong effects of soil pH, Flavobacterium became the main bacteria in soil of TPP-treated plots (i.e., soil sample TPP16) after 6 years. In comparison, the bacteria in VEE-IPBP-treated plots (i.e., soil sample VEE16) were mainly significantly affected by the soil moisture content after 6 years (p < 0.001), and Lysobacter and Bacillus became the main genera composing the soil bacterial community structure in these plots. These findings further demonstrate the effects of soil pH adjusted by earthworms and of the aquatic environment by aquaculture of swamp eels on soil bacterial communities in VEE-IPBP-treated plots.
Conclusions
In this study, a VEE-IPBP system was established through the combination, allocation and regulation of farmland environmental factors, which directly or indirectly affected soil bacterial diversity and community composition. Compared with alterations in soil bacterial Chao, Shannon, and Ace indices, changes in soil dominant bacterial genera such as Lysobacter accounted for significantly enhanced suppression of soil-borne diseases and maintained soil health. Moreover, the effect was more significant as the duration increased. Overall, earthworms alter the microbial activity and pH of soil, increase soil aeration by burrowing, alter the form and content of soil as well as nutrients, and directly or indirectly affect soil bacterial diversity and community structure. At the same time, the inclusion of eels also directly or indirectly affects changes in soil bacterial diversity and community structure, but the mechanism requires further study. In addition, the coexistence of water and drought increased the soil moisture content and also affected physical and chemical reactions, the growth of plants through microclimate, and the structure and abundance of soil bacteria.
Materials and Methods
General information on the experimental site
The experimental site is located in Sanxing Township (31°41′15″N, 121°54′00″E) on Chongming Island within the municipal area of Shanghai, China. Having risen above the sea surface more than 1,300 years ago, Chongming Island, the largest estuarine alluvial island in the world, has a smooth terrain with an average elevation of 4 m; the soil type on the island is silty saline. Situated in a region with a northern subtropical monsoon climate with prevailing south-easterly winds, Chongming Island is warm and wet with ample sunshine and has four distinct seasons with wet and hot summers and cold and dry winters. Typhoons, storms and droughts are common calamitous weather conditions. Chongming Island has an annual mean precipitation of 1,003.7 mm, with most of the precipitation occurring in April through September, an annual mean temperature of 15.3 °C, an annual mean accumulated temperature of 2,559.60 °C for days with temperature ≥10 °C, a frost-free period of 229 d and an annual sunshine duration of 2,104.0 h.
Experimental design
The experiment began in June 2010. Two planting systems, namely, a traditional planting systems (TPP) and VEE-IPBP, were used41. Each planting systems was implemented in three replicate plots. The plots were randomly arranged in a randomized block. Each VEE-IPBP-treated plot consisted of dry and wet fields in the same space. Dry-farmed vegetables were planted, and earthworms were reared in the soil; swamp eels were reared in ditches (Fig. 5). The specific preparations are described as follows.
Vegetable field ditch and plot layout restructuring
In each plot, the vegetable field was surrounded by 100-cm-wide soil ridges at the borders. The ditches were excavated at the borders of and within each plot. Each ditch had an upper opening width of 60 cm, a depth of 60 cm and two sloping sides with an inclination of 60° relative to the ground. Each plot was 600 cm in width. A 40-cm-wide, 20-cm-tall soil ridge was prepared in the centre of each plot. Purse seines were placed along the periphery of the vegetable field to prevent the eels from escaping. In addition, 15% of each mu of the field was covered by water.
Ditch disinfection and plot fertilization
The newly excavated ditches in the vegetable field were disinfected with quicklime spray. For each hectare of the water surface, 225 kg of quicklime was used. After the quicklime was sprayed, the ditches were filled with water, and the water surface was controlled to remain 15–25 cm below the plot surface. A commercial organic fertilizer consisting of OM (413.4 g∙kg−1), nitrogen (N) (17.1 g∙kg−1), phosphorous pentoxide (12.4 g∙kg−1) and potassium oxide (12.3 g∙kg−1) was applied as base fertilizer at a dose of 18 t∙hm−2, and a compound fertilizer (15-15-15) (90% as base fertilizer and 10% as a topdressing material) was evenly sprayed at a dose of 375.0 kg∙ha−2 onto the vegetable field surface. The same commercial organic fertilizer was applied as base fertilizer at a dose of 15 t∙ha−2, and compound fertilizer (15-15-15) (60% as base fertilizer and 40% as topdressing) was evenly sprayed at a dose of 750 kg∙ha−2 onto the cauliflower field surface.
Earthworm and swamp eel introduction and vegetable planting
Earthworms (each weighed 3 g) were introduced at a density of 120 per m2 (a natural density of 60–80 per m2 of the surrounding vegetable field). The earthworm species used is Pheretima guillelmi (Michaelsen,1895), a native species of Chongming Island. In the course of the experiment, new earthworms were born, and old earthworms were killed or eaten by eels. In late winter and early spring, when the temperature was above 6–10, the number of earthworms in the field was investigated, with more being removed or less added to achieve a density of approximately 120 per square metre. The results of the investigation in 2016 showed a density of 155/m2 in treated soil and 66/m2 in non-cultivated soil. Eel fry (40 eels per kg) were introduced at a density of 225 kg∙ha−2. Each season after vegetable harvesting, the earthworm and swamp eel densities were examined and adjusted to the initial values. Taro and cauliflower were intercropped each year. After April 5th every year, Monopterus albus in the furrows of 5 square metres were caught using an eel catch to record density and quality. The taro, cauliflower, earthworm and eel yields and economic benefits are shown in Table 3, with higher values than in the traditional planting model.
Soil sample collection
Before the experiment began in June 2010, reference soil samples were collected from the vegetable field. These samples are denoted as TPP10-1, TPP10-2 and TPP10-3. Soil samples were also collected from TPP- and VEE-IPBP-treated plots after crop harvesting in November 2013 and November 2016, respectively. The soil samples collected from TPP-treated plots in 2013 and 2016 are denoted as TPP13-1, TPP13-2 and TPP13-3 and TPP16-1, TPP16-2 and TPP16-3 respectively. The soil samples collected from VEE-IPBP-treated plots in 2013 and 2016 are denoted as VEE13-1, VEE13-2 and VEE13-3 and VEE16-1, VEE16-2 and VEE16-3, respectively. All soil samples were collected from the surface layer (0–20 cm) using a stainless steel soil sampler in an “S”-shaped pattern. In each replicate plot, soil samples were collected from 15 different locations and then mixed and placed in a sealed polyethylene bag, which was subsequently stored in a low-temperature preservation box and returned to the laboratory. In the laboratory, impurities (plant and animal residues) were removed, after which the soil samples were sieved through a 20-mesh sieve. Some soil samples were dried and analysed for determination of basic physical and chemical properties; others were subjected to high-throughput sequencing analysis.
Test items and methods
Determination of physical and chemical properties of soil. The physical and chemical indices for the soil, including OM, TN, TP, TK, AN, AP, AK and moisture contents and pH, were determined according to protocols of Analytical Methods of Soil Agricultural Chemistry42.
Illumina high-throughput sequencing analysis. Soil samples stored in a box filled with dry ice were transported to Shanghai Biozeron Biotechnology Co., Ltd. for Illumina (rapid-mode, 250-bp, paired-end) high-throughput sequencing analysis. The 515F–907 R primer set (primer sequences: 515 F: GTGCCAGCMGCCGCGG; 907 R: CCGTCAATTCMTTTRAGTTT) was used to amplify the V4-V5 regions of the bacterial 16 S rDNA. Three biological replicates were performed for each soil sample. An ABI GeneAmp® 9700 was used for polymerase chain reaction (PCR) with the following parameters: a) 1 × (5 min at 95 °C); b) 27 × (30 s at 95 °C; 30 s at 55 °C; 45 s at 72 °C); c) 1 × (10 min at 72 °C; 10 °C until halted by user).
Data processing
Analysis of soil physical and chemical properties
One-way analysis of variance was performed using SPSS 13.0 to analyse differences in physical and chemical properties of soil between TPP- and VEE-IPBP-treated plots.
OTU clustering analysis
The biological information for OTUs with 97% similarity was statistically analysed based on the USEARCH algorithm43.
Bacterial diversity analysis
Species richness and diversity indices for bacterial communities were analysed using QIIME44.
Species composition analysis
Based on datasheets in tax_summary (a document folder), plots were produced using the R language to analyse the distribution of communities in soil samples at phylum and genus levels.
Difference analysis
Communities or species leading to a significant difference in sample partitioning were determined using the LDA effective size (LEfSe)45,46.
Correlation analysis
The canonical correspondence analysis (CCA) algorithm in the vegan package in R software was used to produce plots and analyse relationships among environmental factors, samples and bacterial communities or those between any two environmental factors, samples and bacterial communities47.
Sequencing data statistics and optimization
Illumina high-throughput sequencing and optimization generated 576,175 sequences from 15 samples obtained from five treatments with different planting systems (TPP or VEE-IPBP) and durations (0, 3 or 6 years) with a total of 216,871,530 bases and an average length of 376.4 bp. The base sequences with a length of 351–400 bp accounted for 99.91% of all sequences (Table 4).
References
Wu, S. Y., Li, F. R., Xu, C. B., Wei, Y. H. & Zheng, X. W. The bird grain support combined with efficient ecological planting mode of seed technology exploration. China Rice. 12, 35–36 (2006).
Liu, C. F., Wu, Y. Y., Zhang, W. J., Guo, L. Q. & Wen, Y. L. The research on emission reducing benefits of combination of planting-breeding in China. Environ. Pollu. & Control. 38, 87–89 (2016).
Lei, C. Q. & Wei, D. J. Discussion on rice duck breeding technology and ecological benefits in Xinjiang rice planting area. Animal husbandry in Xinjiang. 32, 53–54 (2017).
Qi, Z. Q., Wang, X. Z. & Chen W. Economic benefit analysis of family breeding combined with beef cattle farm. Chinese Journal of Traditional Veterinary Science. 107–107 (2017).
Chen, H. B., Lu, J. D., Zhao, L. & Li Zhao, H. Origin and present development status of circling agriculture. Chin. J. Agric. Resour. Regional Plan. 12, 65–68 (2007).
Halwart, M. & Gupa, M. V. Culture of Fish in Rice Fields. Italy. FAO. (2004).
Kumaresan, A., Pathak, K. A. & Bujarbaruah, K. M. Analysis of integrated animal-fish production system under subtropical hill agro-ecosystem in India: Growth performance of animals, total biomass production and monetary benefit. Trop. Animal Health Prod. 41, 385–391 (2009).
Pant, J. & Demaine, H. & dwards, P. Bio-resource flow in integrated agriculture-aquaculture systems in a tropical monsoonal climate: a case study in northeast thailand. Agr. Syst. 83, 203–219 (2005).
Li, H. F., Zhu, Z. C. & Wang, J. M. Benefit of rice-shrimp-fish-crab farming in paddy field. J. Aquaculture. 1, 4–5 (1997).
Edited by the national agriculture, animal husbandry and fishery plan office. Beijing: Economic Science Press, (1996).
Edwards, P., Pullin, R. S. V. & Gartner, J. A. Research an Education for the Development of Integrated Crop–Livestock–Fish Farming Systems in the Tropics. Agricultural Systems 32, 293–294 (1988).
Yu, G. R. planting system analysis and optimization control method. Agricultural Publishing House. (1991).
Li, S. X. et al. Circular agriculture development models in the suburbs of metropolis Case study of stereo cycling model of vegetable-earthworm-eel. Shanghai Journal of Agricultural Sciences 33, 23–27 (2017).
Kirk, J. L. et al. Methods of studying soil microbial diversity. J. Microbiol. Methods. 58, 169–188 (2004).
Kerri, L., Steenwerth, L. E., Jackson, F. J., Calderón, M. R. & Kate, M. S. Soil microbial community composition and land use history in cultivated and grassland ecosystems of coastal california. Soil Biol. Biochem. 35, 489–500 (2003).
Klemedtsson, L., Berg, P., Clarholm, M. & Schnurer, J. Microbial nitrogen transformations in the root environment of barley. Soil Biol. Biochem. 19, 551–558 (1987).
Singh, J. S., Raghubanshi, A. S. & Srivatava, S. C. Microbial biomass act as a source of plant nutrients in dry tropical forest and savanna. Nature. 338, 499–500 (1989).
Dong, P. L. I., Jie, W. Z. & Chen, L. J. Influence of fertilizing modes of organic agriculture on the soil microbial activities. Chin. J. Eco. Agric. 13, 99–101 (2005).
Kozdrój, J. & Elsas, J. D. V. Response of the bacterial community to root exudates in soil polluted with heavy metals assessed by molecular and cultural approaches. Soil Biol. Biochem. 32, 1405–1417 (2000).
Kell, J. J. & Tate, R. L. Effects of heavy metal contamination and remediation on soil microbial communities in the vicinity of a zinc smelter. J. Environ. Qual. 27, 609–617 (1998).
Tian, C. J., Chen, J. K. & Zhong, Y. Phylogenetic diversity of microbes and its perspectives in conservation biology. Chin. J. Appl. Ecol. 14, 609–612 (2003).
Wardle, D. A., Yeates, G. W., Nicholson, K. S., Bonner, K. I. & Watson, R. N. Response of soil microbial biomass dynamics, activity and plant litter decomposition to agricultural intensification over a seven-year period. Soil Biol. Biochem. 31, 1707–1720 (1999).
Parle, J. N. A microbiological study of earthworm casts J. General Microbiol. 31, 13–22 (1963).
Daniel, O. & Anderson, J. M. Microbial biomass and activity in contrasting soil material after passage through the gut of earthworm Lumbricus rubellus hoffmeister. Soil Biol. Biochem. 24, 465–470 (1992).
Devliegher, W. & Verstraete, W. Lumbricus terrestris, in a soil core experiment: nutrient-enrichment processes (nep) and gut-associated processes (gap) and their effect on microbial biomass and microbial activity. Soil Biol. Biochem. 27, 1573–1580 (1995).
Shan, J. et al. Digestion and residue stabilization of bacterial and fungal cells, protein, peptidoglycan, and chitin by the geophagous earthworm Metaphire guillelmi. Soil Biol. Biochem. 64, 9–17 (2013).
Schonholzer, R., Hahn, D. & Zeyer, J. Origins and fate of fungi and bacteria in the gut of Lumbricus terrestris l. studied by image analysis. Fems Microbiol. Ecol. 28, 235–248 (1999).
Byzov, B. A., Khomyakov, N. V., Kharin, S. A. & Kurakov, A. V. Fate of soil bacteria and fungi in the gut of earthworms. Eur. J. Soil Biol. 43, S149–S156 (2007).
Gomez, B. M., Aira, M., Lores, M. & Dominguez, J. Changes in microbial community structure and function during vermicomposting of pig slurry. Bioresour. Technol. 102, 4171–4178 (2011).
Doan, T. T., Jusselme, D. M., Lata, J. C., Bo, V. N. & Jouquet, P. The earthworm species metaphire posthuma, modulates the effect of organic amendments (compost vs. vermicompost from buffalo manure) on soil microbial properties. a laboratory experiment. Eur. J. Soil Biol. 59, 15–21 (2013).
Ferlian, O. et al. Invasive earthworms erode soil biodiversity: A meta-analysis. Journal of Animal Ecology. 87, 162–72 (2017).
Jiang, X. T. et al. Illumina sequencing of 16s rrna tag revealed spatial variations of bacterial communities in a mangrove wetland. Microbial Ecol. 66, 96–104 (2013).
Hu, F., Liu, M. Q. & Li, H. X. Effect of soil fauna on soil quality and its research prospect. The Tenth National Congress of the Chinese society of Soil Sciences and the Fifth Symposium on Soil and fertilizer exchange across the Taiwan Straits, Shenyang, China. (2004).
Christensen, P. & Cook, F. D. Lysobacter, a new genus of nonfruiting, gliding bacteria with a high base ratio. Int. J. Syst. Bacteriol. 28, 367–393 (1978).
Hayward, A. C., Fegan, N. & Fegan, M. Stenotrophomonas and lysobacter: ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J. Appl. Microbiol. 108, 756–770 (2010).
Haynes, R. J., Fraser, P. M., Piercy, J. E. & Tregurtha, R. J. Casts of aporrectodea caliginosa (savigny) and lumbricus rubellus (hoffmeister) differ in microbial activity, nutrient availability and aggregate stability. Pedobiologia 47, 882–887 (2003).
Abrahamsson, T. R. et al. Low diversity of the gut microbiota in infants with atopic eczema. J. Aller. Clin. Immunol. 129, 434 (2012).
Buchanan, R. E., Gibbons, N. E. & Berger. Study on microbial bacterial identification manual China Institute of translation group. Eighth edition. Beijing: Science Press. 5, 35–36 (1984).
Liu, F. Community structure of rhizosphere microbial in sanjiang plain. Territory Nat. Resour. Stdy. (2013).
Liu, S. X. et al. Analyzing soil microbial community structure diversity from jianhu wetland lakeside zone using pcr-dgge technique. J. of Agro-Environ. Sci. 32, 1405–1412 (2013).
Zheng, X. Q., Li, S. X. & Yuan, D. W. Effects of earthworm tillage on soil nutrients and enzyme activities in vegetable fields. Plan. Nut. and Fertil. Sci. 18, 1184–1191 (2012).
Lu, R. K. Analytical methods of Soil Agricultural Chemistry. China Agr. Sci. Technol. Press, Beijing, China. pp. 147–197 (2000).
Huse, S. M. et al. Exploring microbial diversity and taxonomy using ssu rrna hypervariable tag sequencing. PLoS Genet. 4, e1000255 (2008).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Zhang, C. et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat. Commun. 4, 2163 (2013).
Segata, N., Izard, J., Waldron, L., Gevers, D. & Miropolsky, L. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Sheik, C. S., Mitchell, T. W., Rizvi, F. Z., Rehman, Y. & Faisa, L. M. Exposure of Soil Microbial Communities to Chromium and Arsenic Alters Their Diversity and Structure. PLoS ONE 7, e40059 (2012).
Acknowledgements
We thank our colleagues at the Institute of Eco-Environment and Plant Protection, Shanghai Academy of Agricultural Sciences for their assistance with sample collection and analysis. This work was financially supported by the Shanghai Academy of Agricultural Sciences Program for Excellent Research Teams (SPERT), the Shanghai Agriculture Applied Technology Development Program under the grant (T20170105), and the Project on Transformation of Agricultural Scientific and technological achievements (133919N1400).
Author information
Authors and Affiliations
Contributions
Xianqing Zheng and Weiguang Lv designed the study. Xianqing Zheng, Juanqin Zhang and Shuangxi Li performed the experiments. Xianqing Zheng, Naling Bai and Hanlin Zhang analyzed the data. Xianqing Zheng wrote the manuscript. Ke Song and Weiguang Lv reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, X., Lv, W., Song, K. et al. Effects of a vegetable-eel-earthworm integrated planting and breeding system on bacterial community structure in vegetable fields. Sci Rep 8, 9520 (2018). https://doi.org/10.1038/s41598-018-27923-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27923-y